Method Article
Evaluation of Color Difference in Placenta with Twin Anemia Polycythemia Sequence
In This Article
Summary
In monochorionic twins with twin anemia polycythemia sequence (TAPS), the donor twin and its corresponding placenta share are pale, while the recipient twin and its placental share have a plethoric aspect. Presented here is a protocol to quantify the color difference in maternal side of TAPS placenta after birth.
Abstract
Twin anemia polycythemia sequence (TAPS) occurs in 5% of monochorionic twins and is characterized by large inter-twin hemoglobin differences. The postnatal diagnostic criteria for TAPS are based on hematologic parameters and placental characteristics. Placental examination after birth shows that color of the maternal side between placental territories of the anemic and polycythemic twins is remarkably different. The color difference in TAPS placentas is higher compared to monochorionic placentas with acute peripartum feto-fetal transfusion; thus, this is used as an additional diagnostic criterion for TAPS. Software such as ImageJ enables the computer-based measurement of color intensity in TAPS placentas. However, a detailed method for the calculation of color differences between anemic and polycythemic components of TAPS placentas has not yet been described. The protocol presented here provides a step-by-step method for analyzing color differences in the maternal side of TAPS placentas using ImageJ software.
Introduction
TAPS is a chronic form of feto-fetal transfusion syndrome and characterized by a large inter-twin hemoglobin (Hb) difference without signs of oligo-polyhydramnios sequences1. The main pathogenesis of TAPS is related to the unique placental angioarchitecture, with only a few minuscule anastomoses allowing for chronic and low velocity transfusion from the donor (anemic twin) to recipient (polycythemic twin)2,3,4. Postnatal diagnosis of TAPS is based on hematological tests showing a large Hb difference and large reticulocyte count difference and/or placental injection with dye showing only minuscule anastomoses5. However, reticulocyte count is not always measured at birth, and placental injection is not routinely performed in most fetal medicine centers. An additional diagnostic criterion may be useful to help diagnose cases without reticulocyte count measurements and without placental dye injection. A striking color difference between the pale skin of a donor twin and the plethoric skin of the recipient twin is consistently present in TAPS twins at birth. A similar color difference of the maternal side between placental territories of the anemic and polycythemic twins is also detected upon gross examination.
It has been recently hypothesized that this placental color difference may be used as a simple and accurate method to confirm a TAPS diagnosis after birth6. The quantification of color difference in TAPS placentas is timesaving compared to placental injection. Additionally, quantification of color difference in TAPS placentas is more cost-effective compared to reticulocyte count. The materials used in this method are easily available in clinical settings Therefore, this method provides simple and accurate criteria for the diagnosis of TAPS. The protocol describes a detailed method for the quantification of color differences from the maternal side of monochorionic placentas with TAPS.
Protocol
This protocol was approved by the Leiden University Medical Center Ethics committee, and postnatal examination of human monochorionic twin placentas is a part of standard care at the Leiden University Medical Center. The acquisition of consent for postnatal examination of human monochorionic twin placentas is waived at the Leiden University Medical Center.
1. TAPS placenta collection and gross examination
- Use all human monochorionic twin placentas with TAPS delivered at any gestational age. Keep the human monochorionic twin placenta as intact as possible. Deliver the monochorionic placenta as gently as possible, if manual removal is necessary.
NOTE: After birth, midwives or neonatologists should transfer TAPS placentas into a plastic box and then to the laboratory. - When the first infant is delivered, use one clamp to clamp the umbilical cord to mark the birth order as first. When the other infant is delivered, use two clamps to clamp the umbilical cord to mark the birth order as second.
- Examine the placenta macroscopically, including placenta intactness, types of umbilical cord insertions, number of umbilical vessels, and gross examination of the dividing membrane to confirm the chorionicity. Collect the inter-twin membrane for pathological diagnosis of monochorionicity. The diameter of TAPS placentas is dependent on gestational age.
NOTE: Examination should be performed within 1 week after delivery to avoid the formation of adhesive blood clots on maternal side. - Keep the placenta refrigerated (1–4 °C) in a dry, plastic box until examination, if needed.
2. Preparation of the placental maternal side
- Trim the amniotic membrane along the placental edge using scissors. Pay attention to the vessels on the membrane and if the velamentous cord insertion is present to prevent blood from flowing out of the placental mass.
NOTE: In velamentous cord insertion, vessels from the umbilical cord move to the surface of the amniotic membrane for some distance before attaching to the placental mass. If the “naked” vessels are cut, the blood from placental mass may flow out and make a mess. - Remove blood clots carefully to prevent damage to the placental lobes.
- Organize the placental lobes on the maternal side to eliminate the gap between lobes. Placentas usually have around 15 lobes and can be identified on the maternal side.
3. Preparation for documentation
- Place the placenta on a plastic panel with the maternal side facing upwards.
- Expose the maternal side of the placenta to light (uniform distribution using two identical lamps on the left and right sides). Use lamps that have a function for adjusting light intensity.
- To reduce reflection, take digital photos with a camera (Table of Materials) in mild light conditions and avoid direct exposure to the light source.
- Take photos perpendicular to the maternal side of placenta.
4. Computerized measurement using ImageJ
- Open ImageJ on the computer by clicking on the software icon.
- Click on “File”, then “Open” to open the placental picture. Once the required image is opened, click on “Image” | “Color”, then “Channels Tool” to choose the “Color” from the drop list.
NOTE: The software can automatically identify the color of the object measured. The placenta is red. In the software, after clicking “Color”, the placental picture will automatically be converted into a red spectrum channel. - Click on “Freehand selections” to respectively contour the plethoric and pale placental share of the polycythemic and anemic twins via visual inspection of different color intensity of the maternal side of the placenta.
- Click on “Analyze” | “Histogram”. Obtain the color intensity histogram after selecting the plethoric area or pale area, respectively. The mode displayed in the intensity histogram represents the color that is most present in the placental area selected.
- Calculate the color difference ratio by dividing the mode of color intensity of the anemic twin by the mode of color intensity of the polycythemic twin.
Results
Placental examination provides valuable information for the diagnosis of TAPS. TAPS placentas are characterized by the presence of few small vascular anastomoses1 (Figure 1, Figure 2). This feature of TAPS placentas is related to the pathogenesis of TAPS and is a postnatal diagnostic criterion. Similar to the color of neonates at birth, the placenta share of the recipient twin in TAPS is typically dark and plethoric, and the placenta share of the donor twin is pale and anemic (Figure 3).
The results show that the measurement of color difference between two placental territories on maternal side is feasible using ImageJ (Figure 4, Figure 5). In the TAPS placenta exemplified in this study, the mode of color intensity histogram in polycythemic and anemic areas is 82 and 209, respectively, yielding a color difference ratio of 2.5 (209/82) (Figure 6). All human monochorionic twin placentas are postnatally examined at the Leiden University Medical Center as a part of standard care for monochorionic twins over the past 25 years. More than 200 TAPS placentas have been examined to confirm the effectiveness of this method.
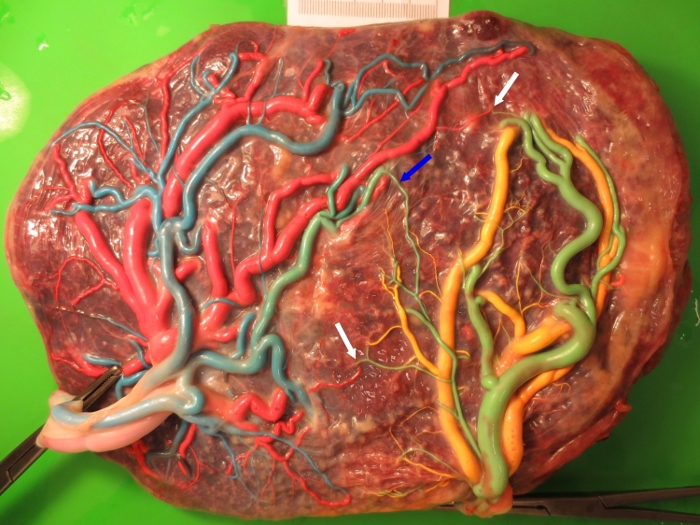
Figure 1: Characteristics of spontaneous TAPS placentas.
Fetal side of a spontaneous TAPS placenta after color dye injection showing the typical angioarchitecture with only two small arteriovenous (AV) anastomoses (white arrows) and one small arterio-arterial (AA) anastomosis (blue arrow). Please click here to view a larger version of this figure.
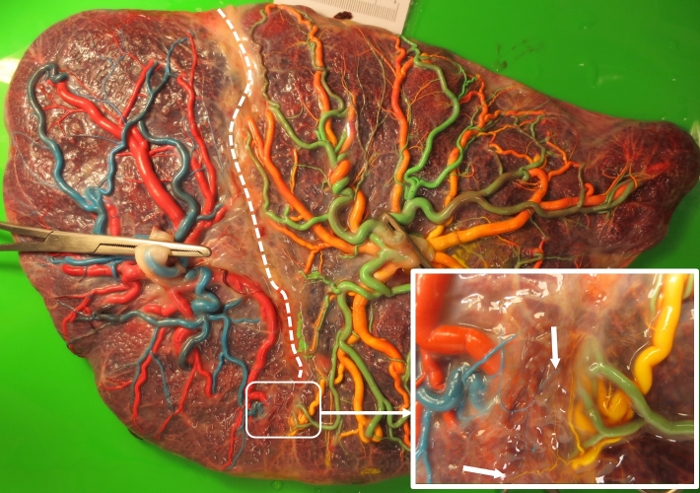
Figure 2: Characteristics of post-laser TAPS placentas.
A post-laser TAPS placenta after color dye injection demonstrating two miniscule residual AV anastomoses. The white, dotted line shows the laser line. The area where the residual anastomoses are localized is enlarged in the inserted picture. The two residual miniscule AV anastomoses are indicated by white arrows. Please click here to view a larger version of this figure.

Figure 3: Color difference in TAPS placentas.
Maternal side of a spontaneous TAPS placenta showing the striking color difference between the two placental shares. Please click here to view a larger version of this figure.
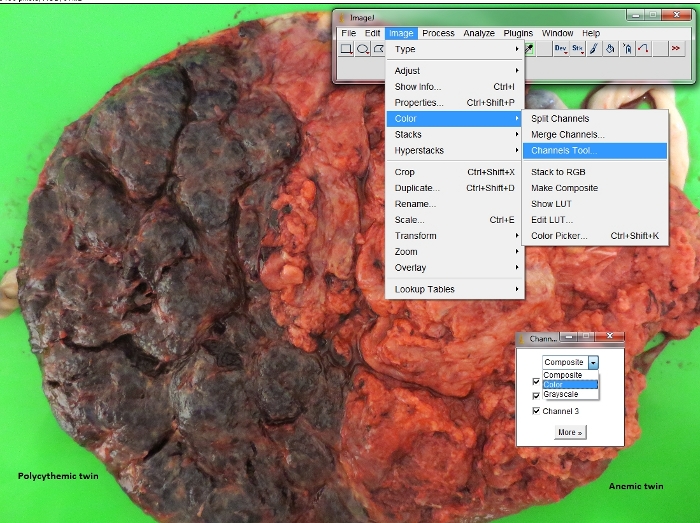
Figure 4: Conversion of color spectrum in placental pictures.
Procedure to convert the digital placental picture into a red spectrum channel in ImageJ. Please click here to view a larger version of this figure.
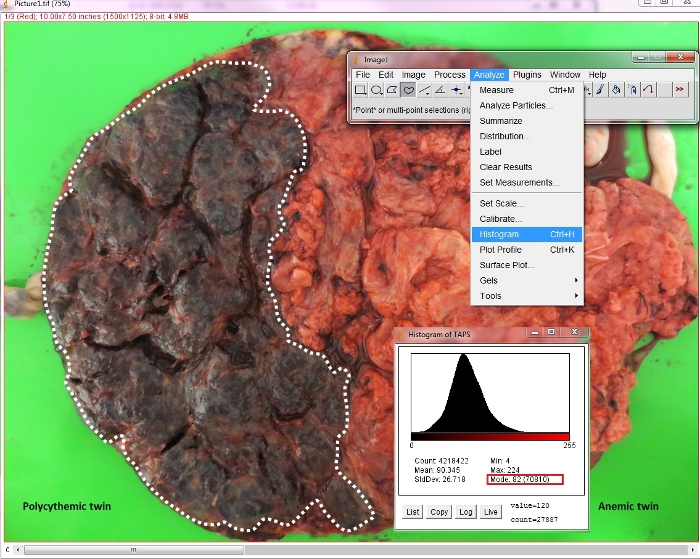
Figure 5: Analysis of the color intensity in each placental share.
Procedure to determine the mode of color intensity histogram of each placental share. Please click here to view a larger version of this figure.
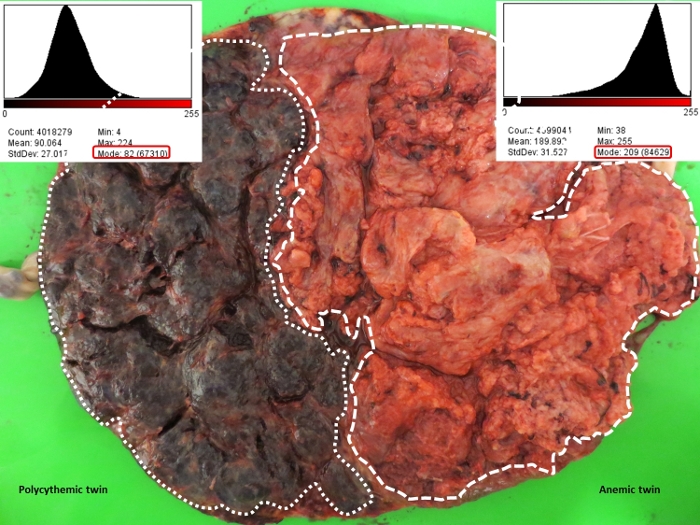
Figure 6: Measurement of color difference ratio in a spontaneous TAPS placenta.
The mode of color intensity histogram of each placental share is shown in the red rectangle. The mode of color intensity histogram in the anemic and polycythemic placental shares is 209 and 82, respectively, yielding a color difference ratio of 2.5 (209/82). Please click here to view a larger version of this figure.
Discussion
Key points to achieving an optimal examination based on our experience include: 1) delivery of the placenta as gently as possible to avoid damage, especially when manual removal is indicated; 2) examination of MC placentas soon after birth to avoid the formation of adhesive clots (preferably within a few days); 3) gentle removal of all blood clots, avoiding damage to the placental lobes; 4) exposure of the maternal side of MC placentas to uniform light conditions and minimization of the light reflection; and 5) taking pictures of the maternal side before dye injection of the fetal side, then quantification of color difference ratio using ImageJ. In this protocol, the ImageJ was used to determine the color difference. Other softwires with function to quantify color intensity are appropriate alternatives. The advantage of ImageJ is its wide application in scientific research and free access. The main limitation of this protocol is the difficulty in setting the identical light intensity and angle for placental pictures. Therefore, it is strongly recommended to use lamps with adjustable light intensity.
All monochorionic placentas have vascular anastomoses connecting the two fetal circulations. These anastomoses may lead to complications such as twin-twin transfusion syndrome (TTTS) or TAPS7,8,9,10. TTTS may occur in 10% of monochorionic twins and is characterized by a large difference in amniotic fluid with oligohydramnios in the donor twin and polyhydramnios in the recipient twin11. TAPS is a newly reported complication that may occur spontaneously in 5% of monochorionic twins or in 13% of TTTS treated with fetoscopic laser surgery. TAPS is characterized by a large inter-twin Hb difference without the degree of amniotic fluid discordances required for the diagnosis of TTTS1,5,12. Prenatal diagnosis of TAPS is currently based on discordant measurements of the middle cerebral artery peak systolic velocity (MCA-PSV) (>1.5 multiples of the median [MoM] in donors and <1.0 in recipients) or a delta MCA-PSV of >0.5 MoM using doppler ultrasound. Postnatal diagnosis of TAPS is based on the presence of large Hb difference (>8 g/dL) and at least one of the following: reticulocyte count ratio (>1.7) or miniscule anastomoses (diameter of <1 mm) detected through colored dye injection5. However, complete hematological tests are not always performed at birth, and reticulocyte count measurements are often missing.
In addition, color dye injection is a complex and time-consuming procedure that is not routinely performed in most centers. Search of additional parameters to help confirm a diagnosis of TAPS is warranted. As shown in a recent study, measuring the color difference between two maternal shares of TAPS placentas can be a useful additional criterion. A cut-off value of 1.5 of the color difference ratio (i.e., ratio of the color intensity in the anemic placental share vs. the plethoric placental share) may be helpful to characterize TAPS placentas6. The presented protocol provides an accurate and simple method to quantify color differences in the maternal side of TAPS placentas. However, larger studies are required to further evaluate the diagnostic value and determine the cut-off value of color differences of the maternal side for postnatal TAPS diagnosis.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (Grant number: 81801465), the Sanming Project of Medicine in Shenzhen (Grant number: SZSM201512012) and National Key Research and Development Program of China (Grant number: 2018YFC1002900).
Materials
| Name | Company | Catalog Number | Comments |
| 20 ml syringes | BD Plastipak | 300613 | |
| Color dye | Royal Talens Schoolverf | 36716010 (blue); 36715010 (green); 36712350 (yellow); 36713570 (pink) | |
| Digital camera | Canon Inc. | ixus 125 hs | |
| ImageJ | National Institute of Health | For Windows ImageJ bundled with 64-bit Java 1.8.0_112 | |
| Umbilical catheters | Vygon | 1270.08 (8 F) 1270.08 (5 F) 1270.04 (4 F) 1270.03 (3.5 F) 1270.02 (2.5 F) |
References
- Lopriore, E., et al. Twin anemia-polycythemia sequence in two monochorionic twin pairs without oligo-polyhydramnios sequence. Placenta. 28 (1), 47-51 (2007).
- Zhao, D., et al. Placental share and hemoglobin level in relation to birth weight in twin anemia-polycythemia sequence. Placenta. 35 (12), 1070-1074 (2014).
- Lopriore, E., et al. Assessment of feto-fetal transfusion flow through placental arterio-venous anastomoses in a unique case of twin-to-twin transfusion syndrome. Placenta. 28 (2-3), 209-211 (2007).
- Lopriore, E., et al. Quantification of feto-fetal transfusion rate through a single placental arterio-venous anastomosis in a monochorionic twin pregnancy. Placenta. 30 (3), 223-225 (2009).
- Tollenaar, L. S. A., et al. Improved prediction of twin anemia-polycythemia sequence by delta middle cerebral artery peak systolic velocity: new antenatal classification system. Ultrasound in Obstetrics and Gynecology. 53 (6), 788-793 (2019).
- Tollenaar, L. S., et al. Color Difference in Placentas with Twin Anemia-Polycythemia Sequence: An Additional Diagnostic Criterion. Fetal Diagnosis and Therapy. 40 (2), 123-127 (2016).
- Zhao, D. P., et al. Prevalence, size, number and localization of vascular anastomoses in monochorionic placentas. Placenta. 34 (7), 589-593 (2013).
- De Paepe, M. E., Burke, S., Luks, F. I., Pinar, H., Singer, D. B. Demonstration of placental vascular anatomy in monochorionic twin gestations. Pediatric and Developmental Pathology. 5 (1), 37-44 (2002).
- Lewi, L., et al. Placental sharing, birthweight discordance, and vascular anastomoses in monochorionic diamniotic twin placentas. American Journal of Obstetrics and Gynecology. 197 (6), 581-588 (2007).
- Robertson, E. G., Neer, K. J. Placental injection studies in twin gestation. American Journal of Obstetrics and Gynecology. 147 (2), 170-174 (1983).
- Lewi, L., et al. The outcome of monochorionic diamniotic twin gestations in the era of invasive fetal therapy: a prospective cohort study. American Journal of Obstetrics and Gynecology. 199 (5), (2008).
- Slaghekke, F., et al. Fetoscopic laser coagulation of the vascular equator versus selective coagulation for twin-to-twin transfusion syndrome: an open-label randomised controlled trial. Lancet. 383 (9935), 2144-2151 (2014).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved