Method Article
Establishing a Murine Superficial Bladder Cancer Model via an Intravesical Cell Administration Technique
In This Article
Summary
This protocol describes a unique method for constructing an orthotopic model of superficial bladder cancer.
Abstract
This study presents an innovative method for establishing an orthotopic murine bladder tumor model with high efficiency and precise tumor localization. After anesthetizing female C57BL/6J mice in a supine position, a 24 G intravenous needle is inserted into the bladder to evacuate its contents. A 34 G dispensing needle is then introduced through the catheter, rotated five times to create a focal injury to the bladder dome mucosa, and subsequently removed. The MB49 cell suspension is aspirated and connected to a 30 G dispensing needle, which is inserted into the bladder via the catheter. Tumor cells are injected submucosally into the bladder under pressure. This technique results in minimal trauma to the mice, a high tumor take rate, and a fixed tumor location. It is characterized by simplicity and excellent reproducibility. This model provides an ideal experimental platform for developing intravesical therapies for bladder cancer, facilitating the advancement and optimization of treatment strategies for this malignancy.
Introduction
Bladder cancer represents a significant global health burden, with notable sex-specific disparities in incidence and prognosis. This malignancy is characterized by distinct molecular subtypes, each associated with diverse pathogenic pathways. The molecular and pathological features differ significantly between non-muscle invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC)1,2. NMIBC accounts for approximately 75% of cases, featuring tumors of transitional epithelial origin that remain localized without metastasis or spread. Conversely, MIBC is characterized by the infiltration of cancer cells into the bladder's muscle layer, accompanied by a high risk of dissemination, frequently necessitating cystectomy in clinical practice3.
In preclinical research, models that accurately represent the superficial stage of the disease are essential for evaluating drug therapies. The establishment of a superficial bladder carcinoma in situ model is, therefore, of paramount importance, serving as a critical research tool for the development of clinical drugs and innovative instillation therapies.
The development of murine orthotopic bladder cancer models has traditionally faced challenges. Chemically induced models often begin as superficial tumors but may evolve into invasive forms, with variability that limits their utility in large-scale experiments4. Traditional orthotopic implantation models, which rely on the injection of tumor cells through the bladder wall5, struggle to faithfully reproduce superficial bladder cancer. Alternative methods, including mucosal injury combined with tumor cell instillation6,7,8,9,10, have been attempted but are limited by high mortality rates and low tumor take rates, hindering their broader application.
This study aims to develop a new method for constructing a superficial bladder cancer model that demonstrates greater stability, lower mortality, and fixed tumor positioning compared to existing models. By achieving a more precise representation of the disease in its early stages, the current model is poised to enhance the rigorous evaluation of preventive and therapeutic interventions, thereby advancing bladder cancer treatment.
Protocol
All animal experimental protocols and procedures were approved by the Ethical Review Committee for Animal Experimentation of Tianjin Medical University, Tianjin, China (approval number SYXK: 2020-0010). Six- to eight-week-old female C57BL/6J mice were used for this study. The animals were housed under controlled environmental conditions, including a 12-h light-dark cycle, temperatures ranging from 21-25 °C, adjustable humidity levels between 30%-70%, and unrestricted access to food and water unless otherwise specified. The details of the reagents and equipment used are listed in the Table of Materials.
1. Cell preparation
- Culture the murine bladder cancer cell line MB49 in complete Dulbecco's Modified Eagle Medium (DMEM), supplemented with 10% fetal bovine serum (FBS) to support optimal growth.
- Maintain the cell cultures under standard conditions at 37 °C in a humidified incubator with 5% CO2. Refresh the media every 2-3 days to ensure cell health and viability.
- Harvest the cells using a standardized trypsin digestion procedure. Gently pipette to dislodge the cells from the culture flask after applying trypsin and incubating for a brief period.
- Count the harvested cells using a hemocytometer to determine the exact cell density. Prepare a single-cell suspension by resuspending cells to 2 x 103/µL in phosphate-buffered saline (PBS). Keep this suspension on ice to maintain cell viability.
2. Animal preparation
- Group house the mice (5 mice per cage) in individually ventilated polycarbonate cages.
- Allow the mice to acclimate for 7 days before the start of the study.
3. Animal orthotopic tumor model generation
- Administer anesthesia to the mice via intraperitoneal injection using a 2.5% solution of Avertin (following institutionally approved protocols).
- Wait until the mice reach an appropriate level of anesthesia for surgery, as indicated by a decrease in respiratory rate with an increase in depth, the absence of eyelid and corneal reflexes, reduced muscle tone and reflex responses, and no reaction to a tail pinch.
- Position the anesthetized mouse in a supine position to facilitate the procedure.
- Use a 24 G intravenous catheter with the needle stylet removed for the urethral catheterization procedure. Apply ample lubrication to the catheter to minimize discomfort and facilitate smooth insertion.
- Identify the urethra, situated immediately posterior to the vulvar folds and anterior to the vagina, while keeping the mouse in a supine position.
- Begin the catheterization by approaching the urethra at a 45-degree angle to navigate beneath the pubic bone. Adjust to a more shallow angle to guide the catheter through the urethra and into the bladder.
- Avoid applying excessive force against resistance, as this can lead to urethral or bladder perforation. Confirm the catheter's location within the bladder lumen by checking for urine within the catheter.
- Gently apply pressure to the lower abdomen of the mouse to facilitate the drainage of urine from the bladder. Ensure the bladder is completely emptied to prepare for the subsequent steps of the procedure.
- Gently push the 24 G intravenous catheter toward the top of the bladder. Ensure the tip of the catheter makes contact with the bladder's apex.
- Once contact is confirmed, release the catheter, allowing it to retract slightly. Maintain the catheter in a fixed position relative to the mouse to ensure stability and prevent dislodgement.
- Insert the modified 34 G dispensing needle along the 24 G catheter. Advance the needle until it reaches the top of the bladder.
- Once positioned, rotate the needle six times to create a localized mucosal injury at the bladder's apex. After completing the rotation, carefully withdraw the needle along the catheter.
- Connect the 30 G dispensing needle to the top of a 1 mL syringe. Ensure a secure connection between the needle and the syringe.
- Once connected, draw the pre-prepared 2 x 10³/µL MB49 cell suspension into the syringe by gently pulling back the plunger. Verify that the cell suspension is aspirated into the syringe without any air bubbles.
- Insert the 30 G dispensing needle along the 24 G catheter. Ensure the needle is properly aligned and secured within the catheter.
- Once positioned, apply pressure to the plunger of the 1 mL syringe to inject the contents (prepared in step 1). Maintain steady pressure on the plunger for 60 s to ensure the contents are delivered without significant volume reduction.
- After 60 s, carefully withdraw the syringe and the 30 G dispensing needle from the catheter.
4. Post-operative monitoring
- Place the anesthetized mouse in a supine position on a heating pad until the righting reflex returns.
- Ensure the animal is not left unattended until it has regained sufficient consciousness.
- Do not return the animal to the company of other animals until it has fully recovered.
Results
The efficacy of submucosal injections was initially assessed through the administration of Trypan Blue. Post-injection, the dye's distribution within the submucosal layer was clearly visualized, confirming the precise and controlled delivery of the injected substances (Figure 1).
Tumor development was meticulously tracked. Approximately 14 days post-implantation, a notable incidence of hematuria was observed, a critical symptom indicative of bladder neoplasia. The tumors were identified as firmly established within the submucosal layer of the bladder. The early growth pattern of these tumors was characterized by expansive, non-invasive expansion, consistent with the classification of NMIBC. Histological examination confirmed the absence of muscular invasion, with tumors confined to the submucosal layer, thereby aligning with the criteria for NMIBC (Figure 2).
Macroscopic observations further corroborated the tumor formation process. The tumors were visually distinct, exhibiting papillary growth with a range of sizes and shapes (Figure 3). Additionally, the tumors grew relatively slowly, providing sufficient time for research in the experiment (Figure 4).
In this study, to verify the tumor location and the absence of urethral seeding, we used MB49-luc cells for implantation, followed by small animal imaging. Figure 5 shows the in vivo bioluminescence images taken 35 days after tumor cell injection, clearly demonstrating that the tumor is located in the bladder with no evidence of ectopic seeding, such as in the urethra.
Tumor tissue typically becomes detectable 14 days post-implantation. In our experience, 70% or more of the mice exhibit hematuria by day 14. The procedure generally results in no mortality. Without intervention, euthanasia becomes necessary by day 60. Euthanasia criteria include lethargy, cachexia, ruffled fur, hunching, unresponsive behavior, or a ≥20% loss of initial body weight.

Figure 1: Efficacy of submucosal injection with trypan blue. Demonstration of submucosal injection efficacy using Trypan Blue. Arrows indicate the injection sites where residual Trypan Blue remains visible even after three washes with PBS. Please click here to view a larger version of this figure.

Figure 2: Histological confirmation of tumor implantation. Histological confirmation of tumor implantation in the submucosa via H&E staining. Scale bar: 50 µm. Please click here to view a larger version of this figure.

Figure 3: Direct visualization of bladder tumors. Direct visualization of bladder tumors, highlighting evident vascular proliferation at the tumor base. Scale bar: 2 mm. Please click here to view a larger version of this figure.
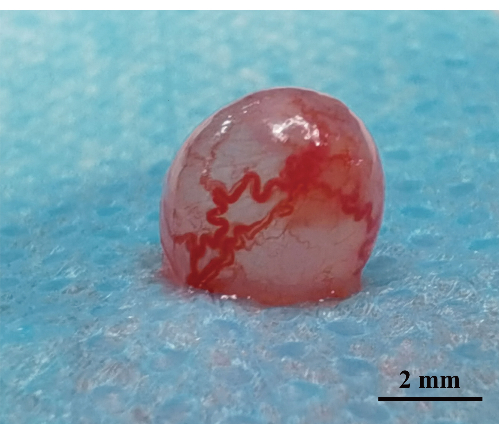
Figure 4: Tumor status on day 35 post-implantation. Assessment of tumor status on day 35 post-implantation. The bladder wall at the tumor base exhibits enlarged and disorganized blood vessels, accompanied by a broadly extensive tumor base. Scale bar: 2 mm. Please click here to view a larger version of this figure.

Figure 5: In vivo bioluminescence imaging of bladder cancer. In vivo bioluminescence images of mice 35 days after implantation with MB49-LUC bladder cancer cells. The images are color-coded to represent fluorescence intensity: red indicates high fluorescence (high tumor activity), and blue indicates low fluorescence (low tumor activity). Regions of Interest (ROI) were selected in the bladder area to quantify the bioluminescence signal specific to the tumor. The results demonstrate that tumors are localized in the bladder with no evidence of ectopic seeding, such as in the urethra. Low signal regions may be due to scattered signals from high-intensity areas penetrating through the tissue and do not necessarily indicate the presence of tumor cells. Please click here to view a larger version of this figure.

Figure 6: Diagram of intravesical administration of cancer cells. Schematic representation of the intravesical administration of cancer cells to construct the orthotopic model of superficial bladder cancer. Please click here to view a larger version of this figure.

Figure 7: Configuration of modified dispensing needles. Configuration of modified dispensing needles within the 24 G catheter. (A) Photograph of the 34 G dispensing needle within the 24 G catheter. (B) Photograph of the 30 G dispensing needle within the 24 G catheter. Please click here to view a larger version of this figure.
Discussion
Research on bladder cancer relies on animal models, which are indispensable for both basic and applied research. To better mimic the tumor growth environment, orthotopic bladder tumor models provide a superior approach compared to subcutaneous tumor models11.
Currently, orthotopic bladder cancer models can be categorized based on experimental purposes into two main types: those utilizing immunodeficient mice, such as patient-derived xenograft (PDX) models1 and cell line-derived xenograft (CDX) models5, and those utilizing immunocompetent mice, such as syngeneic engraftment models12.
The PDX model involves implanting patient-derived tumor tissues into immunodeficient mice, allowing for personalized assessment of the tumor's response to chemotherapeutic drugs. However, the lack of an intact immune system in these mice presents significant limitations for studying immune-related mechanisms. Similarly, the CDX model, which uses human-derived tumor cell lines, offers high tumor formation rates but also suffers from the absence of an immune microenvironment5.
In the context of immunocompetent mice, the primary method for constructing orthotopic bladder tumors involves injecting murine-derived MB49 cell lines into the bladder wall to form in situ bladder tumors12. This procedure requires laparotomy and intravesical injection of tumor cells, posing challenges such as potential tumor cell leakage and needle tract seeding. Consequently, it is difficult to create superficial bladder tumors, which hampers the accurate replication of the progression of human bladder cancer from non-muscle-invasive to muscle-invasive stages13.
To establish superficial orthotopic bladder tumors, traditional modeling techniques typically involve inducing mucosal injury using agents such as acid-base solutions or electrocautery, followed by the intravesical instillation of tumor cell suspensions, with retention times of 2 h or more8,14. However, these methodologies present several significant limitations. Firstly, they fail to ensure uniformity in the number and location of tumor implants within the bladder, thereby compromising the reproducibility of the model. Furthermore, these techniques often result in severe bladder damage, leading to high mortality rates. The resultant bladder scarring and reduced capacity pose substantial obstacles for studies on novel therapeutic agents that necessitate repeated intravesical instillations. This not only undermines the reliability of experimental outcomes but also raises ethical concerns regarding animal welfare.
Therefore, developing an animal model that can accurately simulate superficial human bladder cancer would provide a better experimental platform for understanding tumor proliferation and progression. Such a model would be invaluable for researching new intravesical therapeutic agents and advancing our knowledge of bladder cancer biology.
This study delineates an in situ implantation method that employs a blunt-tipped dispensing needle to inject tumor cells submucosally under pressure (Figure 6). The study utilizes the MB49 cell line and female C57BL/6J mice for constructing an in situ tumor model.
During the construction of this model, the protective layer on the normal bladder mucosal surface poses a significant challenge for direct tumor cell instillation, often preventing effective tumor formation8. Consequently, it is necessary to induce localized injury at the bladder dome using a 34 G blunt needle to create a suitable "soil" for tumor cell implantation.
The 34 G and 30 G dispensing needles used in this study have undergone partial modifications. The tip of the 34 G needle has been clamped to create a slightly irregular end, enhancing its capability to cause mucosal damage. The connection points of both the 34 G and 30 G needles have been trimmed to ensure that the exposed portions on the 24 G catheter are 0.3 mm to 0.5 mm (Figure 7A) and 1 mm to 1.5 mm (Figure 7B), respectively.
This entire procedure can be completed within 10 min, eliminating the need for laparotomy or prolonged urethral ligation for intravesical instillation. However, this approach also has limitations. Due to the multiple technical steps involved in this modeling method-such as inserting the indwelling catheter through the urethra into the bladder and rotating the 34 G blunt needle to achieve the appropriate degree of mucosal injury without perforating the bladder wall-experimenters must practice to enhance the success rate of tumor implantation. Additionally, if excessive force is used during mucosal injury or tumor cell injection, it can lead to bladder perforation or tumor implantation into the muscle layer.
In conclusion, this technique offers significant advantages over traditional methods, including reduced procedural time, lower mortality, and improved consistency in tumor localization and growth, without the need for invasive procedures or prolonged intravesical retention. These benefits make it a valuable tool for preclinical studies aimed at evaluating new therapeutic agents for bladder cancer.
Disclosures
The authors declare that they have no competing interests.
Acknowledgements
This study was supported by the Tianjin Municipal Health Industry Key Project fund (grant no. TJWJ2022XK014), the Scientific Research Project fund of Tianjin Municipal Education Commission (grant no. 2022ZD069), the Tianjin Institute of Urology Talent Funding Program (grant no. MYSRC202310), the Clinical Medicine Research Project fund of the Second Hospital of Tianjin Medical University (grant no. 2023LC03), the Youth Fund of the Second Hospital of Tianjin Medical University (grant no. 2022ydey15), and the Talent Cultivation Project of the Department of Urology, the Second Hospital of Tianjin Medical University (grant no. MNRC202313). The sponsors played a role in the preparation, review, and approval of the manuscript.
Materials
| Name | Company | Catalog Number | Comments |
| 34 G dispensing needle | Suzhou Haorun Fluid Technology Co., Ltd., Suzhou, China | SGL-362 | |
| 30 G dispensing needle | Suzhou Haorun Fluid Technology Co., Ltd., Suzhou, China | SGL-362 | |
| Avertin | Sigma-Aldrich LLC | T48402 | |
| Trypan blue | Sigma-Aldrich LLC | 302643 |
References
- Tran, L., Xiao, J. F., Agarwal, N., Duex, J. E., Theodorescu, D. Advances in bladder cancer biology and therapy. Nat Rev Cancer. 21 (2), 104-121 (2021).
- Dyrskjøt, L., et al. Bladder cancer. Nat Rev Dis Primers. 9 (1), 58 (2023).
- Sim, W. J., et al. C-met activation leads to the establishment of a TGFβ-receptor regulatory network in bladder cancer progression. Nat Commun. 10 (1), 4349 (2019).
- Overdevest, J. B., et al. CD24 expression is important in male urothelial tumorigenesis and metastasis in mice and is androgen-regulated. Proc Natl Acad Sci U S A. 109 (51), E3588-E3596 (2012).
- Theodorescu, D., Cornil, I., Fernandez, B. J., Kerbel, R. S. Overexpression of normal and mutated forms of HRAS induces orthotopic bladder invasion in a human transitional cell carcinoma. Proc Natl Acad Sci U S A. 87 (22), 9047-9051 (1990).
- Cohen, S. M. Comparative pathology of proliferative lesions of the urinary bladder. Toxicol Pathol. 30 (6), 663-671 (2002).
- Ninalga, C., Loskog, A., Klevenfeldt, M., Essand, M., Tötterman, T. H. Cpg oligonucleotide therapy cures subcutaneous and orthotopic tumors and evokes protective immunity in murine bladder cancer. J Immunother. 28 (1), 20-27 (2005).
- Chade, D. C., et al. Histopathological characterization of a syngeneic orthotopic murine bladder cancer model. Int Braz J Urol. 34 (2), 220-226 (2008).
- Chan, E. S., et al. Optimizing orthotopic bladder tumor implantation in a syngeneic mouse model. J Urol. 182 (6), 2926-2931 (2009).
- Kasman, L., Voelkel-Johnson, C. An orthotopic bladder cancer model for gene delivery studies. J Vis Exp. 82, e50181 (2013).
- Puzio-Kuter, A. M., et al. Inactivation of p53 and PTEN promotes invasive bladder cancer. Genes Dev. 23 (6), 675-680 (2009).
- Tu, M. M., et al. Targeting DDR2 enhances tumor response to anti-PD-1 immunotherapy. Sci Adv. 5 (2), eaav2437 (2019).
- Li, G., et al. Fluorinated chitosan to enhance transmucosal delivery of sonosensitizer-conjugated catalase for sonodynamic bladder cancer treatment post-intravesical instillation. ACS Nano. 14 (2), 1586-1599 (2020).
- Watanabe, T., et al. An improved intravesical model using human bladder cancer cell lines to optimize gene and other therapies. Cancer Gene Ther. 7 (12), 1575-1580 (2000).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved