Method Article
Improving Outcomes in Anteromesial Temporal Lobe Resections - A Demonstration of Resecting the Temporal Piriform Cortex
In This Article
Summary
Here, we demonstrate an approach to intraoperative neurosurgical guidance in anteromesial temporal lobe resections, specifically highlighting the use of tractography and anatomical masks to aid safe resection of the temporal portion of the piriform cortex - an area increasingly regarded as a crucial surgical target in drug-resistant mesial temporal lobe epilepsy.
Abstract
Anteromesial temporal lobe resection (ATLR) is a useful treatment option for drug-resistant mesial temporal lobe epilepsy (DRmTLE). Growing evidence suggests the piriform cortex plays a crucial role in the generation and propagation of seizures in DRmTLE - and that the resection of the temporal portion of the piriform cortex is associated with significantly improved rates of seizure freedom.
Here, we present the resection of the temporal portion of the piriform cortex in ATLR, using high-resolution preoperative probabilistic tractography algorithms and fused anatomical masks of the structures of interest into the intraoperative neuronavigation and microscope head-up display (HUD).
All patients undergoing comprehensive preoperative assessment and investigations for DRmTLE provided informed, written consent to record an intraoperative video of the procedure. Patients were identified by an expert multidisciplinary team of epileptologists, epilepsy neurosurgeons, neuropsychologists, neuropsychiatrists, and electrophysiologists at a large epilepsy surgery center. The preoperative imaging pipeline included the delineation of critical structures. This included the temporal piriform cortex, and high-resolution probabilistic tractography for essential tracts at risk (e.g., optic radiation and inferior fronto-occipital fasciculus). These were co-registered to the preoperative volumetric neuronavigation scan and uploaded to the intra-operative neuronavigation system.
Presented here is a step-by-step procedure of ATLR, including the resection of the temporal portion of the piriform cortex. The protocol combines Advanced structural and diffusion MR imaging and intraoperative visual aids to integrate anatomical masks of critical grey matter structures and white matter tracts into the surgical workflow in the operating room.
Introduction
Anteromesial temporal lobe resection (ATLR) is the most effective treatment for drug-resistant mesial temporal lobe epilepsy (DRmTLE)1,2, with 50%-70% seizure freedom rates and relatively low morbidity3,4,5. The procedure has also been shown to improve quality of life6,7,8, employment rates5, and psychosocial wellbeing9.
The canonical ATLR, described by Spencer et al.10, involves resection of the temporal pole, uncus, amygdala, hippocampus, parahippocampal gyrus, and fusiform gyrus. Critical white matter pathways involved in vision (the optic radiation, in particular, Meyer's loop11,12) and language (e.g., the inferior fronto-occipital fasciculus13 and the arcuate fasciculus14,15) are at risk of injury when accessing the temporal horn of the lateral ventricle. The following protocol outlines an approach to avoiding these white matter tracts using high-resolution preoperative probabilistic tractography and fused anatomical masks of the structures of interest into the intraoperative neuronavigation and microscopic head-up display (HUD).
The field's traditional understanding is that maximal hippocampal resection is beneficial to maximize the rates of postoperative seizure freedom. However, recent voxel-wise analyses of post-ATLR cases demonstrate that the resection of the temporal portion of the piriform cortex in ATLR greatly increases the chance of seizure freedom. They also showed that there was no association between posterior hippocampal resection and seizure freedom16,17. Accordingly, it has been proposed to update Spencer's technique by limiting the hippocampal resection to the anterior 55% of the hippocampus, in language-dominant hemisphere ATLRs, to preserve memory function16,18.
While there has been increasing interest in the use of novel minimally invasive therapies, particularly laser interstitial thermal therapy (LITT), surgical resection remains the standard of care for drug-resistant focal epilepsy1, and the efficacy of LITT has been shown to produce a lower proportion of Engel 1 seizure outcomes (58%-59%)1,19 compared to ATLR (60%-70%)3,4,5,20, and so is still an area requiring further investigation21.
There is a growing body of evidence supporting the hypothesis that the piriform cortex (Figure 1) is a critical region in the propagation and/or epileptogenesis of seizures in adults16,17,22,23,24 and children25 with mesial temporal lobe epilepsy. The piriform cortex is a ribbon of three-layered allocortex (similar to the arrangement of hippocampal cortex) that is draped around the entorhinal sulcus mesial to the temporal stem26,27, and therefore forms the confluence of the temporal and frontal lobes. It can, therefore, easily be considered as consisting of frontal and temporal divisions, described in detail in the literature22,25,28,29,30.
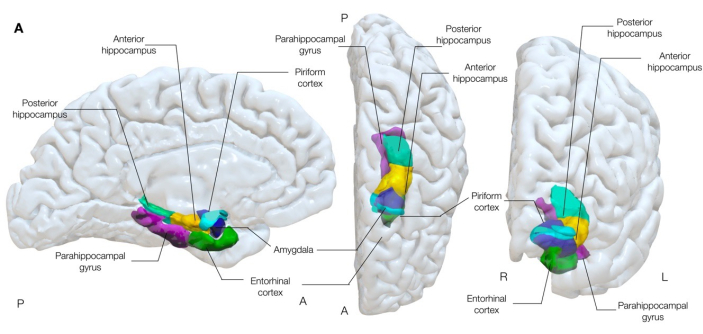
Figure 1: Semi-transparent 3-dimensional rendering of mesial temporal structures of the brain. This figure demonstrates the anatomical associations of the piriform cortex (cyan) to surrounding mesial temporal lobe anatomy. Left medial, center superior, and right anterior views. Please click here to view a larger version of this figure.
The piriform cortex is supero-mesial to the amygdala and has long been implicated in animal studies to be a common node in networks that disseminate epileptogenic discharges31-33, and generates seizures following electrical stimulation more easily than neighboring mesial structures, including the amygdala and hippocampus34. Its position, with extensive connections to the entorhinal, limbic, orbitofrontal, and insular cortices, as well as to the thalamus, olfactory bulb, amygdala, and hippocampus, also lends itself to a role as a key propagation pathway of epileptogenic discharges in focal epilepsy30.
EEG-fMRI and positron emission tomography (PET) studies further support an important role of the piriform cortex in DRmTLE, showing interictal activation, and reduced γ-Aminobutyric acid type A (GABAA) receptor binding in the piriform cortex is associated with increased seizure activity35,36,37.
Two significant recent imaging studies in DRmTLE have shown that postoperative seizure freedom is associated with a greater extent of resection of the piriform cortex; Galovic et al. demonstrated in a large retrospective cohort that removal of at least half of the piriform cortex improved the odds of becoming seizure-free by a factor of 16 (95% CI, 5-47; p < 0.001)17. It was also demonstrated that the resection volumes of other mesial temporal structures were not associated with seizure freedom, a finding replicated and supported by the voxel-wise analyses performed by Sone et al., who showed that only piriform cortex resection in left TLE was associated with seizure freedom16 (Figure 2).
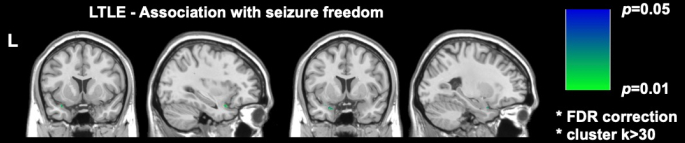
Figure 2: Voxel-wise association with postoperative seizure freedom in left TLE. The only area significantly correlated with seizure freedom is the temporal portion of the piriform cortex, p = 0.01 (green in coronal and sagittal T1-weighted MRI slices). Adapted from Sone et al.16 with permission. Please click here to view a larger version of this figure.
Borger et al. also demonstrated in a large retrospective cohort that only the proportion of resected temporal piriform cortex is associated with improved rates of seizure freedom both at 1 year3 and at longer follow-up (mean 3.75 years)23. They further corroborated that the volume resected of the hippocampus and amygdala did not predict seizure freedom.
The importance of the piriform cortex being disconnected from the aberrant epileptogenic network in mTLE has also been demonstrated in LITT, with Hwang et al. showing at 6-month follow-up that percent piriform cortex ablation was associated with ILAE class 1 outcomes38 (OR 1.051, 95% CI 1.001-1.117, p = 0.045), but that this was a trend that was not significant at 1 year5. This seems to support the emerging data regarding LITT, that there is a positive, but potentially less permanent, improvement in seizure outcomes, which has led to LITT being used commonly as a "first-stage" procedure, with resective surgery offered to those in whom seizure freedom is not achieved by LITT.
There is, therefore, strong evidence that resection of the temporal portion of the piriform cortex as a key target in achieving seizure freedom in drug-resistant mesial temporal lobe epilepsy. However, as the retrospective cohort from Galovic et al. demonstrated, this ribbon of entorhinal cortex is in a difficult location to target surgically when performing an ATLR, meaning if it is not directly targeted, it is not always successfully removed. We show in this study how to safely target and resect the temporal portion of the piriform cortex as part of an ongoing prospective surgical study, to assess its impact on improving seizure freedom rates postoperatively39.
The focus of the following protocol is on the technical aspects of the image acquisition and processing, the surgical approach, and how we ensure resection of the temporal portion of the piriform cortex in ATLR, while integrating high-resolution preoperative probabilistic tractography and fused anatomical masks of the structures of interest into the intraoperative neuronavigation and microscope head-up display (HUD). The protocol also uses a specific planning software platform40, which allows 3-dimensional viewing and integration of multimodal imaging for surgical review and planning, and a neuronavigation system that allows integration with the operative microscope (specifics are detailed in the Table of Materials).
Protocol
These methods and protocols are part of an ongoing prospective surgical trial that was approved by the Health Research Authority on 10/09/2020, Research Ethics Committee (REC) London reference: 20/LO/0966. The protocol was prospectively registered: ISRCTN72646265, on 25/09/2020, is available online39, and has been presented at a national conference41.
The following protocol is applied to all patients undergoing ATLR for DRmTLE in patients 18-70 years old (the age group of patients operated upon for this indication at our specialist adult epilepsy surgery center), all operated upon by the same surgeons (AWM, AM). All participants provided informed consent prior to inclusion in the study. All participants underwent thorough preoperative evaluation and investigations directed by the expert surgical epilepsy multidisciplinary team at the authors' comprehensive epilepsy surgery center, consisting of neurosurgeons, epilepsy neurologists, neuropsychologists, psychiatrists, neuroradiologists, and other members of the specialist epilepsy therapies services. Prior to surgery, all had up-to-date volumetric T1, T2, and FLAIR MRIs as outlined in the protocol below, as well as standard preoperative blood and a review by the neuro anesthesia team, ensuring that they were safe to proceed to surgery under general anesthesia. The commercial details of the reagents and the equipment used in this study are provided in the Table of Materials.
1. Imaging acquisition and processing
NOTE: High-resolution presurgical, 3-month, and 1-year postsurgical magnetic resonance imaging (MRI) scans are routinely acquired in people undergoing epilepsy surgery at our center. MRI data were acquired between March 2020 and March 2024 on the same MRI scanner for consistency. The standardized image acquisition and many of the processing components have been previously described in the literature and are referenced appropriately in the protocol summarized below:
- Acquire the following MRI sequences:
- A standard 3D isometric (1 mm) T1-weighted sequence with inversion-recovery fast spoiled gradient recalled (IR-FSPGR) echo [echo time (TE) 3.1 ms, repetition time (TR) = 7.4 ms, inversion time = 400 ms, field of view (FOV) = 224 × 256 × 256 mm, matrix = 224 × 256 × 256, voxel size = 1.00 × 1.00 × 1.00 mm = 1.00 mm3, parallel imaging acceleration factor = 2] and;
- A coronal dual-echo fast recovery fast spin echo proton-density/T2-weighted sequence used for T2 relaxometry42 (TE = 30/119 ms, TR = 7600 ms, FOV = 220 × 220 mm, matrix = 512 × 512, slice thickness = 4 mm, voxel size = 0.43 × 0.43 × 4.00 mm = 0.74 mm3, SENSE factor = 2).
- Use the above T1-weighted sequence as input to the geodesic information flows (GIF v3) algorithm to parcellate the brain into 162 anatomical regions using NiftyWeb43.
- Produce anatomical masks of the structures of interest using the GIF parcellations (parahippocampal gyrus, fusiform gyrus, create hippocampal profiling information, and segment the hippocampus into the anterior 55% and posterior 45% using Hipposeg44), overlay these in the planning software on the patient's volumetric preoperative imaging (as in step 1.1).
- Perform automated piriform cortex segmentation and split the piriform cortex into frontal and temporal components from the volumetry techniques explained in previous work from our lab17,45,46. Once generated, overlay these masks on the patient's preoperative imaging.
- Acquire the following Diffusion MRI images:
- A standard resolution multi-shell acquisition (2 mm isotropic resolution, 11, 8, 32, and 64 gradient directions at b-values 0, 300, 700, 2500 s/mm2) and;
- A high-resolution multi-shell acquisition (1.6 mm isotropic resolution, 101 directions, 14 b0, b-values: 300, 700 and 2500 s/mm2).
- Correct the acquired diffusion data using MRtrix3 (https://mrtrix.org)47 for:
- Noise using "dwidenoise" in MRtrix348.
- Signal drift49.
- Gibbs-ringing using "mrdegibs" in MRtrix350.
- Distortion using a reverse phase encoding gradient with FSL TOPUP algorithm (https://fsl.fmrib.ox.ac.uk/fsl51).
- Eddy currents and movement artifacts using FSL's eddy algorithm (https://fsl.fmrib.ox.ac.uk/fsl)52, rotating the b vectors53.
- Bias field using the ANTs algorithm (https://mrtrix.org47,54).
- Use multi-shell, multi-tissue Constrained Spherical Deconvolution (CSD)55 to estimate response functions for white and grey matter, and cerebrospinal fluid (CSF).
- Perform Anatomically Targeted Automated Tractography to reconstruct fiber bundles of interest12,56,57: the optic radiation, inferior fronto-occipital fasciculus (IFOF; language dominant cases), and middle longitudinal fasciculus (MLF; language non-dominant cases). Follow the steps below.
- Extract cortical termination points for each fiber bundle and group them into seed and termination ROI.
- Create exclusion cortical regions and ROI using non-terminating cortical regions.
- Perform anatomically constrained tractography58 via the probabilistic fiber tracking algorithm iFOD259 using hybrid surface and volume segmentation in MRtrix347 selecting a maximum of 5,000 streamlines from 300 million seeds.
- Perform the tractography in step 1.8.3 twice for each fiber bundle, switching the seed and termination ROI.
- Convert the resultant fiber bundles to probabilistic maps, thresholded at a value of 0.01 - use these as an additional exclusion criteria to remove spurious streamlines.
- Review the resulting fiber bundles (dilated by 2 mm) and anatomical masks in the planning software to ensure they are anatomically accurate (this step is performed by two epilepsy neurosurgeons in our unit).
- Spatially register the resulting anatomical grey matter and white matter tract masks to the reference T1-weighted MR image in step 1.1 and loaded to the intraoperative navigation system in the intra-operative MRI (iMRI) operating room.
- Acquire T1, T2, and T2-FLAIR weighted volumetric MRI images immediately prior to surgery (< 24 h) with skin fiducials on the patient's scalp. Co-register these images to one another, and the T1 weighted reference image and masks described above, and demonstrated in Figure 3.
- Register" the patient on the neuro-navigation system with a surface-matching laser and smart-pointer.
- Verify the accuracy of the image co-registration and patient with two epilepsy neurosurgeons prior to the case beginning to ensure accuracy.
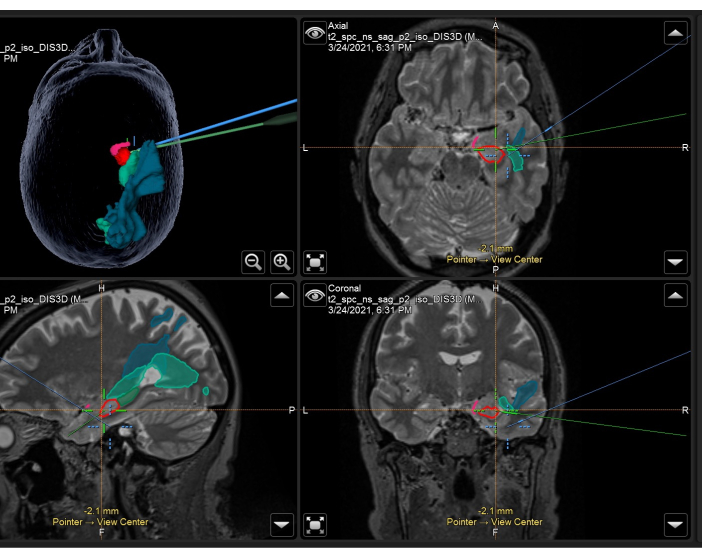
Figure 3: Screenshot of neuronavigation system demonstrating the volumetric T2-weighted MRI with overlaid anatomical masks and tracts used intraoperatively in a right ATLR. Top left panel: 3-dimensional reconstruction of the patient's head, demonstrating anatomical masks. Top right: axial, Bottom left: sagittal, and bottom right: coronal views also showing overlaid anatomical masks on T2-weighted volumetric MRI scan. Anatomical masks displayed: temporal portion of the piriform cortex (pink), anterior 55% of the hippocampus (red), posterior 45% of the hippocampus (dark green, only seen on sagittal image), optic radiation (mid-green), middle longitudinal fasciculus (blue). The blue crosshair is the integrated position of the focus of the microscope, and the green crosshair is the position of the neuronavigation pointer being used within the surgical field. Please click here to view a larger version of this figure.
2. Surgical technique
NOTE: The below steps summarize practice in the authors' center and is not intended to be an exposition of the only surgical approach to an ATLR, rather a demonstration of how the authors have standardized the approach to this procedure to provide reliable and reproducible resections, including resection of the temporal piriform cortex.
- Positioning and approach
- Following standard preoperative checks and safety measures, position the patient supine, elevate the ipsilateral shoulder with a roll, and turn the head to the contralateral side.
- Flex the head laterally to ensure the malar eminence is the highest point of the surgical field, and fix in pins (3-point skull fixation device - see examples, including those compatible with intraoperative [iMRI] theaters in the Table of Materials).
NOTE: The positioning is crucial to allow adequate access to the mesial and posterior temporal lobe structures, particularly the lateral flexion, to ensure the malar eminence is the highest point of the surgical field. - Infiltrate local anesthetic to pin sites and skin incision.
- Register the patient space to the neuronavigation system using a combination of skin fiducials and surface tracking (specific to the brand of neuronavigation system utilized). Confirm registration accuracy against palpable or visible bony/other anatomical landmarks.
- Mark the hairline and the root of the zygoma (identified by manual palpation). Identify and mark the course of the Sylvian fissure using the neuronavigation, and the planned curvilinear frontotemporal 'question mark' skin incision.
- Remove hair along the planned skin incision using electric hair clippers.
- Perform a frontotemporal incision from 1 cm anterior to the tragus, curving in a question mark fashion, to prevent injury to the frontotemporal branch of the facial nerve. Identify and preserve the superficial temporal artery if possible. Positioning and marking of skin incision are shown in Figure 4.
- Cover and secure the wound edges with antimicrobial-soaked mastoid swabs and Raney clips.
- Incise the temporalis muscle and elevate it either in a myocutaneous or interfascial/subfascial flap (preventing injury to the frontotemporal branch of the facial nerve).
- Wrap the skin flap in antimicrobial solution-soaked gauze and retract it anteriorly (avoiding using metal securing instruments if in iMRI theaters).
- Create 2 burr holes in the cranium with a perforator drill: the first just above the root of the zygoma to maximize inferior exposure, the second inferior frontal to facilitate visualization of the Sylvian fissure.
- Perform a standard frontotemporal craniotomy60,61,62, exposing the superior and middle temporal gyrus 1 cm above the Sylvian fissure.
- Drill away the anterior and inferior margins of the craniotomy to allow easy access to the middle temporal fossa floor as well as the temporal pole anteriorly. If mastoid air cells are encountered, seal these with bone wax and fibrin glue both on encountering them and at the end of the procedure prior to closing layers superficial to the bone.
NOTE: It is important to ensure the craniotomy allows access to the floor of the middle cranial fossa, as this will be the approach to the temporal horn of the lateral ventricle via the collateral sulcus. It is also important to thoroughly seal off any encountered mastoid air cells to prevent a postoperative CSF leak and resultant symptoms and potential infections. - Open the dura in a U-shaped fashion with the base reflected anteriorly and extended in a stellate fashion. Hitch the dura away from the operative field with sutures (such as 3-0 silk).
- Release CSF from the middle cranial fossa floor and anterior aspect of the middle cranial fossa.
NOTE: The CSF release step is important to allow sufficient working space to surgically access the middle cranial fossa floor and collateral sulcus from below without retraction and strain on the basal temporal lobe.
- Lateral neocortical removal
- Coagulate the pia of the middle and inferior temporal gyri (MTG and ITG, respectively) in a line perpendicular to the skull base and in line with the anterior projection of the temporal horn of the lateral ventricle (confirmed on neuronavigation system). Tailor the anterior-posterior extent of this line on a case-by-case basis dependent on the presurgical evidence of lateral neocortical involvement in seizure onset/propagation.
NOTE: The anterior-posterior extent of the lateral neocortical resection should take into account the preoperative evidence (or lack thereof) of its involvement in seizure propagation, and this is tailored for the individual patient. - Employ a transcortical approach in the ITG to expose the middle cranial fossa floor and identify the collateral sulcus, which is lateral to the parahippocampal gyrus and medial to the fusiform gyrus.
- Coagulate the pia of the superior temporal gyrus (STG) anterior to the above lateral neocortical incision, parallel to the direction of the Sylvian fissure. This is perpendicular to the line described in step 2.16, and extends anteriorly to the temporal pole.
- Develop the plane between STG and Sylvian fissure using subpial dissection techniques, protecting the branches of the middle cerebral artery, and carry this dissection down to the horizontal part of the Sylvian fissure to the level of the inferior limiting sulcus of the insula.
NOTE: Care should be taken to maintain the integrity of the pia of the STG bordering the Sylvian fissure while performing the subpial dissection in step 2.2.4, as this protects the branches of the middle cerebral artery in the fissure, as well as other structures such as the oculomotor nerve and posterior communicating artery (step 2.2.14) and the optic tract (step 2.3) later in the operation. - Integrate the microscope with the neuronavigation system.
- Confirm the accuracy of the object overlays of the anatomical masks described in section 1 and visualize these on the microscope HUD.
- Slant the posterior resection line anteriorly superiorly to minimize the resection of STG, and extend it progressively through the MTG and ITG, through the fusiform gyrus to the collateral sulcus.
- Check the relationship of the posterior resection margin against the neuronavigation system with the optic radiation (OR) mask visualized.
- Visualize the maximal anterior extent of the OR mask on the microscope and ensure that it is posterior to the resection margin on the microscope HUD to ensure no damage to the OR, which would result in a visual field deficit postoperatively, as demonstrated in Figure 5.
NOTE: Step 2.2.9 is a critical step to prevent a postoperative visual field deficit due to damage to Meyer's loop of the optic radiation. If there is a lot of brain shift and the surgeon would like to further minimize the risk of damage to the optic radiation, or if the projections on the HUD of the microscope are not working correctly, the authors suggest using the neuronavigation pointer to aim the approach to the anterior-most tip of the temporal horn of the lateral ventricle, as this minimizes the risk of OR injury. - Follow the collateral sulcus superiorly until the temporal horn is encountered (confirm this on the neuronavigation system), as shown in Figure 6. The wall of the temporal horn can be identified by blue-tinged ependyma.
NOTE: In individuals in whom the pathology is not hippocampal sclerosis, and the hippocampal head is bulky, the ventricle can be difficult to find. In these cases, a troubleshooting option is to use the neuronavigation pointer on the base of the medial temporal fossa where there is no shift to find where the tip of the ventricle is projecting. It is normally helpful to look for this on a coronal plane. If the ventricle can't be found, we suggest to first remove the pole and then find the ventricle with the ultrasonic aspirator. - Divide the basal temporal leptomeninges lateral to the temporal horn exposure.
- Open the ventricle anteriorly to expose the hippocampal head (as demonstrated in Figure 6).
- Extend the posterior resection margin to join the disconnection to the ventricle and this allows the disconnection of the neocortical block.
- Mobilize the temporal pole following the line of dissection on the margin of the tentorial edge. Take care not to perform the disconnection over the edge of the tentorium to avoid injuries to mesial structures, including the oculomotor nerve and the posterior communicating artery.
NOTE: To reduce the risk of damaging structures in the cisterna cruralis, follow the shape of the edge of the tentorium without going over the edge of the tent. The uncus will remain in place and can be taken as a separate specimen.
- Coagulate the pia of the middle and inferior temporal gyri (MTG and ITG, respectively) in a line perpendicular to the skull base and in line with the anterior projection of the temporal horn of the lateral ventricle (confirmed on neuronavigation system). Tailor the anterior-posterior extent of this line on a case-by-case basis dependent on the presurgical evidence of lateral neocortical involvement in seizure onset/propagation.
- Mesial temporal resection, including the temporal piriform cortex
- Clear the tissue of the uncus with dissection and use of the ultrasonic aspirator mesially until the oculomotor nerve and posterior communicating artery are visible. Halt the extent of posterior resection when the pes (the most mesial extent of the hippocampal head) is visualized.
- Perform the resection of the amygdala with the ultrasonic aspirator, limited superiorly by the pia of the endorhinal sulcus, and until the optic tract is visualized, and mesially by the pial plane of the basal cisterns.
NOTE: Take care to preserve the temporal stem and not enter the frontal lobe when performing step 2.3.2. At times, due to brain shift, the neuronavigation may overestimate the extent of resection mesially; in this regard, the resection is safe to continue until the optic tract is visualized through the pia, and this represents the mesial boundary of the resection. This is also described in Usui et al.63The dorsal margin of the amygdala is commonly described as approximated by an imaginary line connecting the choroidal point to the proximal middle cerebral artery bifurcation64. - Ensure the temporal portion of the piriform cortex is resected by removing any residual tissue via subpial dissection progressing inferiorly from the temporal side of the Sylvian fissure until the vein of the inferior circular sulcus of the insula is visualized. This is a similar resection margin to that described by Usui et al. when resecting amygdalar-uncal lesions63.
NOTE: If the uncus (in step 2.3.1) or piriform cortex are difficult to remove (at times, they can be very adherent to the pia mater), the authors suggest the use of a Rhoton dissector rather than the ultrasonic aspirator to minimize injury to the pia mater. - Confirm resection of the temporal portion of the piriform cortex using the anatomical mask described in step 1.4 overlaid on the microscope HUD, as demonstrated in Figure 7.
- Hippocampal resection
- Place linteens (or similar broad cottonoids) to retract the choroid plexus (and anterior choroidal artery) mesially to facilitate visualizing the fimbria hippocampi.
- Disconnect the fimbria hippocampi from its arachnoid attachment, exposing the hippocampal sulcus that carries the Ammon's horn arterial vascular arcade.
- Disconnect the hippocampus from its tail (either limiting this resection to the anterior 55% of the hippocampus in language dominant hemisphere resections in an attempt to minimize verbal memory deficits as outlined in16, or extend as posteriorly as the level of the tectum of the midbrain in non-language dominant hemisphere resections).
- Disconnect the hippocampal head from the pes hippocampi. Coagulate the hippocampal arterial arcade as required.
- Remove the disconnected hippocampus en bloc.
- Perform subpial dissection of the parahippocampal gyrus and subiculum, and ensure hemostasis in the resultant surgical cavity. Remove the pes hippocampi, visualizing and protecting the brainstem.
NOTE: Care should be taken when performing step 2.4.7, as there is no protective pial boundary between the pes and the brainstem at this level.
- Intraoperative imaging and closure
- Remove all metallic items from the surgical field, cover the wound, and perform an intraoperative MRI scan, typically including volumetric T1, T2, FLAIR, and DWI sequences.
- Review the intraoperative imaging with a consultant neuroradiologist alongside two epilepsy consultant neurosurgeons, to ensure the desired amount of mesial temporal lobe structures (including the temporal portion of the piriform cortex, as well as the variable amounts of hippocampus as outlined above in step 2.36) have been successfully resected. Also review the DWI imaging to ensure no areas of ischemia have been caused during the procedure.
- Once the above is confirmed, transfer the patient back to the operating table, and confirm hemostasis in the surgical cavity at normotension for the patient.
- Close the wound in a standard fashion, replacing the bone flap, securing this with plates and screws at 3 points, and a standard closure of the muscle, fascia, and skin layers with sutures, leaving a surgical wound drain in place for 24 h.
NOTE: Postoperative care is in the authors' centre, typically in the neurosurgical high-dependency unit for the first 24 h, followed by a step-down to a neurosurgical specialist ward when the high-dependency unit feels this is appropriate. The patients are monitored for their neurological observations, have postoperative blood including urea and electrolytes, full blood count and differentials, and are typically discharged approximately 72 h postoperatively. They are then followed up as an outpatient in 4-6 weeks, and at 3-4 months and 1 year postoperatively as a minimum with interval imaging.
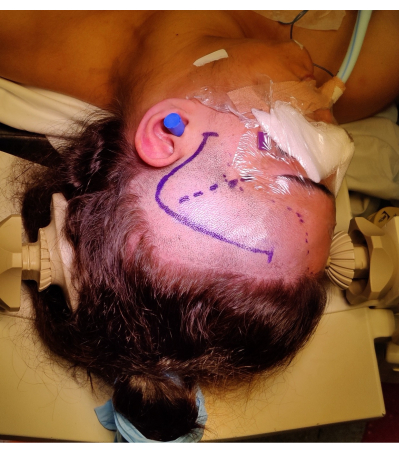
Figure 4: Image of the positioning of the patient for a right ATLR, demonstrating marking of the 'question mark' right frontotemporal skin incision, hairline, and Sylvian fissure. Not pictured is the left shoulder roll under the patient's left shoulder to allow the angle of the positioning of the head without placing undue strain on the patient's neck, and not impeding venous return. Images were captured and included with the consent of the patient. Please click here to view a larger version of this figure.
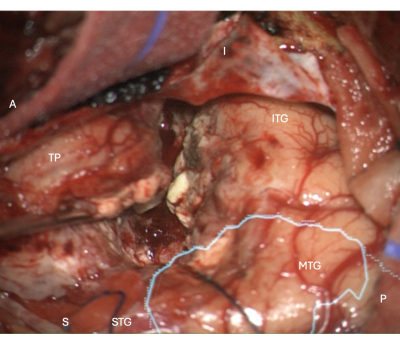
Figure 5: Intraoperative image from the microscope demonstrating the lateral neocortical resection margin in a right ATLR, with the overlaid anatomical mask of the optic radiation (cyan) - demonstrating the resection margin is anterior to the OR. Labels demonstrate the orientation of the operative view: A = anterior, P = posterior, I = inferior, S = superior, STG = superior temporal gyrus, MTG = middle temporal gyrus, ITG = inferior temporal gyrus, TP = Temporal Pole. Please click here to view a larger version of this figure.
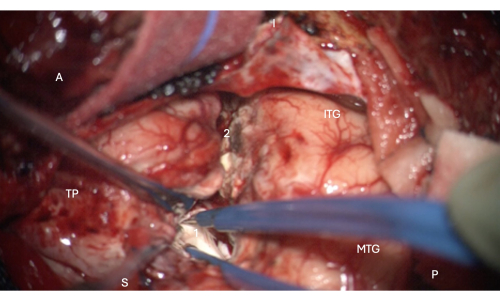
Figure 6: Intraoperative image from the microscope demonstrating entry into the anterior portion of the temporal horn of the lateral ventricle, showing the hippocampal head within it (pale white, 1). Labels: A = anterior, P = posterior, I= inferior, S = superior, MTG = middle temporal gyrus, ITG = inferior temporal gyrus, 2 = lateral neocortical resection margin, following the collateral sulcus superiorly at the depth to find the temporal horn of the lateral ventricle, TP = Temporal Pole. Please click here to view a larger version of this figure.

Figure 7: Intraoperative image from the microscope demonstrating HUD overlay of the anatomical mask of the temporal portion of the piriform cortex (pink outline, labeled Pi). This figure demonstrates complete resection - there is no remaining brain tissue, only the pial boundary of the endorhinal sulcus mesial to the resection, protected in this image with the overlying longitudinal patty in the image, just above the central white crosshair of the microscope HUD. Labels: A = anterior, P = posterior, I = inferior, S = superior, STG = Superior temporal gyrus, MTG = middle temporal gyrus, ITG = inferior temporal gyrus, FL = frontal lobe, SV = Sylvian veins (overlying the Sylvian fissure), Pi = temporal portion of piriform cortex. Please click here to view a larger version of this figure.
Results
This protocol and the surgical techniques have been applied within an ongoing study, interrogating the effects of temporal piriform cortex resection and its impact on seizure freedom following ATLR for DRmTLE. The aim of this study is to prospectively determine whether seizure freedom after removal of the temporal piriform cortex does indeed improve seizure freedom in DRmTLE, as the growing body of retrospective data in the literature suggests.
To date, we have employed the described protocol in 36 consecutive patients undergoing ATLRs, all of which were assessed by a consultant neuroradiologist and two experienced epilepsy neurosurgeons for whether the temporal portion of the piriform cortex was resected on the volumetric T1-weighted intraoperative MRI scan obtained as part of the protocol described. In 100% of cases, the temporal portion of the piriform cortex was resected successfully, confirmed by a neuroradiologist, and in no cases was further resection in that region required to ensure adequate removal of the target tissue. There were also no immediate intra- or postoperative complications or deficits such as significant bleeding, infarcts, or strokes due to damage to cortical vessels and deeper vessels (such as the branches of the middle cerebral artery, or the posterior communicating artery, which runs alongside the resection boundaries), nor any injury to cranial nerves which are in close proximity to the resection area (particularly the oculomotor and trochlear nerves, as well as the ipsilateral optic tract), confirmed by the neuroradiologist.
Figure 8 and Figure 9 provide examples of these successful resections, demonstrating the anatomical mask of the temporal piriform cortex derived from the preoperative MRI overlaid on the neuronavigation system onto the intraoperative MRI scan once the resection has been performed. These images demonstrate complete resection of the temporal portion of the piriform cortex in both left (Figure 8) and right-sided (Figure 9) resections
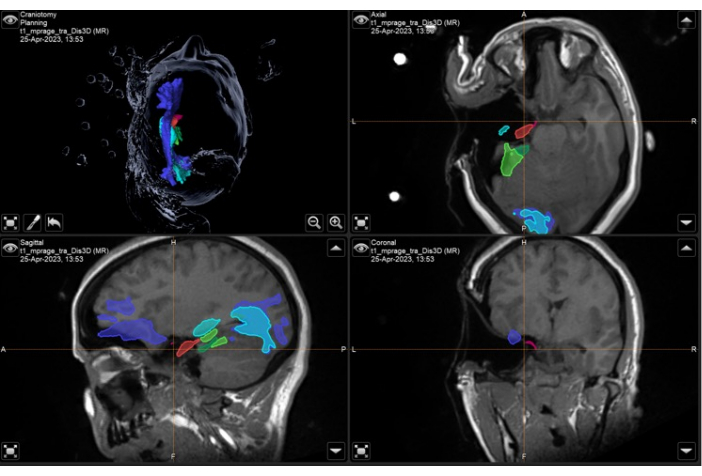
Figure 8: Screenshot of neuronavigation system demonstrating the intraoperative volumetric T1-weighted MRI with overlaid anatomical masks and tracts demonstrating complete resection of the left temporal portion of the piriform cortex (pink). Top left panel: 3-dimensional reconstruction of the patient's head, demonstrating anatomical masks. Top right: axial, bottom left: sagittal, and bottom right: coronal views also showing overlaid anatomical masks on T1-weighted volumetric MRI scan. Anatomical masks displayed: temporal portion of the piriform cortex (pink), anterior 55% of the hippocampus (red), posterior 45% of the hippocampus (light green, only seen on sagittal image), optic radiation (cyan), inferior fronto-occipital fasciculus (purple-blue), anterior 55% of the parahippocampal gyrus (dark green), posterior 45% of the parahippocampal gyrus (light green). The orange crosshair is the position of the images in the 3 planes displayed. There is an expected brain shift visible in these images, given there has been a significant resection of the left temporal lobe, and the intraoperative images have been taken with the patient in the surgical position with the left malar eminence as the highest point (as described in the protocol). Please click here to view a larger version of this figure.

Figure 9: Screenshot of neuronavigation system demonstrating the intraoperative volumetric T2-weighted MRI with overlaid anatomical masks and tracts demonstrating complete resection of the right temporal portion of the piriform cortex (pink). Top left panel: 3-dimensional reconstruction of the patient's head, demonstrating anatomical masks. Top right: axial, bottom left: sagittal, and bottom right: coronal views also showing overlaid anatomical masks on T2-weighted volumetric MRI scan. Anatomical masks displayed: temporal portion of the piriform cortex (pink), anterior 55% of the hippocampus (purple), posterior 45% of the hippocampus (blue), and middle longitudinal fasciculus (green). The orange crosshair is the position of the images in the 3 planes displayed. There is an expected brain shift visible in these images, given there has been a significant resection of the right temporal lobe, and the intraoperative images have been taken with the patient in the surgical position with the right malar eminence as the highest point (as described in the protocol). Please click here to view a larger version of this figure.
This is a positive illustration that our inclusion of intraoperative anatomical grey matter masks and critical white matter tracts, integrated both into the neuronavigation software and the HUD of the microscope, aids in ensuring successful, targeted resection of the area of interest. When this is compared against the retrospective cohort from the same surgeons and team (interrogated in detail in Galovic et al.'s analysis17), in which the seizure-free group had a significantly larger and variable proportion of the piriform cortex resected compared to the non-seizure-free group - the outlined techniques have allowed us to develop and perform a standardized, reliable resection of the temporal portion of the piriform cortex in 100% of prospectively recorded cases.
Discussion
This protocol provides a reliable, targeted resection of the temporal portion of the piriform cortex - posited to be a crucial structure in the epileptogenesis and propagation of the mesial temporal lobe epilepsy network16,17,24,25,30.
Components of the standard ATLR technique we perform at our center are adapted from Spencer et al10, and have been previously described in work from our lab16,17. Details of some of the potential adaptations and alternative approaches to a standard ATLR are provided in a similar step-wise fashion by Al-Otaibi and colleagues65, and this is a useful comparator to the steps we outline in the surgical technique section of the protocol in this study to demonstrate the variety of acceptable variations to practice in this procedure.
We outline in detail both the preoperative planning and image processing required to allow the use of intraoperative, anatomically accurate grey and white matter fiber masks of eloquent structures, and the use of these models intraoperatively to guide resection of the targeted structure during a standardized approach to ATLR. The authors believe this allows consistent, targeted, and safe resection of this difficult to reach supero-mesial part of the mesial temporal lobe - an area of the brain that is not consistently resected in a standard ATLR66. The variability in the extent of temporal piriform cortex resection may be due to the challenging anatomical location to access during the operation. Given the growing evidence from various sources, from animal31,32,33, to structural16,17,22,23 and functional35,36,37, and LITT data24, demonstrating the importance of this area in DRmTLE, we feel this is an important technical advance, and modification of Spencer's described ATLR, to potentially improve seizure freedom rates and minimize language, visual and memory deficits postoperatively.
These results are part of an ongoing prospective cohort study, and as such, the present article and video do not include the primary (seizure freedom) and secondary outcomes of this study (language, visual, and memory deficits), but rather focus on the technical aspects of integrating multimodal imaging, and intraoperative aids into the intraoperative surgical workflow to target and reproducibly and consistently resect the temporal portion of the piriform cortex.
With this in mind, we describe in detail the steps taken to safely remove the temporal portion of the piriform cortex in these patients. We found that when the intra-operative MRI was performed and analyzed in all 36 consecutive ATLRs in this study, targeted resection of the temporal piriform cortex had been successfully completed in all cases, and no further resection was required. It is also important to note that there were no immediate intra- or postoperative complications or deficits such as significant bleeding, infarcts, or strokes due to damage to cortical and deeper vessels, nor cranial nerve injury. We, therefore, consider the methods described above to allow for a reliable resection of the temporal portion of the piriform cortex while minimizing surgical complications.
This method relies on the availability of structural and diffusion MRI to create the described anatomically accurate grey and white matter masks that act as critical structures to preserve or resect during surgery. High-quality, consistent image acquisition and processing capabilities are therefore essential, and this is a crucial step in the implementation of the above protocol to produce reliable preoperative images and masks, as well as the availability of neuronavigation software. This could be considered a limitation of this approach, particularly in resource-limited settings, as the protocol requires more resources than a standard ATLR. We also utilize intraoperative MRI scans to ensure we have achieved the desired boundaries of resection (including the temporal piriform cortex). This was included in the above protocol to be able to validate our results of successful resection of the targeted area, but the use of intra-operative MRI is not essential and can be replaced by a postoperative MRI to assess the extent of resection and immediate postoperative complications, as is standard neurosurgical care, and this is more readily generalized to more resource-limited settings.
We submit that this is a safe, reproducible approach to resecting the temporal portion of the piriform cortex when performing an anteromesial temporal lobe resection for drug-resistant mesial temporal lobe epilepsy, and represents a valuable modification to previously described ATLR techniques that may improve seizure freedom rates. We also demonstrate successful and safe integration of state-of-the-art tractography and anatomical masks into the intraoperative surgical workflow, using intraoperative aids such as neuronavigation systems and head-up displays on the operative microscope to integrate these advanced anatomical masks. The integration of these intraoperative aids into the surgical workflow can easily be expanded to improve the accuracy and reproducibility of other complex or high-risk resections, and the authors use these techniques to guide post-SEEG resections, for example, where specific small targets in the brain are to be targetted.
Disclosures
Authors Debayan Dasgupta and John S. Duncan receive funding from the Wellcome Trust Innovation Program (218380/Z/19/Z). Lawrence P. Binding is supported by Epilepsy Research UK (grant number P1904). The aforementioned authors and Sjoerd B. Vos are partly funded by the National Institute for Health Research University College London Hospitals Biomedical Research Centre (NIHR BRC UCLH/UCL High Impact Initiative BW.mn.BRC10269). The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Acknowledgements
This work was supported by Epilepsy Research UK (grant number P1904) and the Wellcome Trust Innovation Program (218380/Z/19/Z). This work was partly funded by the National Institute for Health Research University College London Hospitals Biomedical Research Centre (NIHR BRC UCLH/UCL High Impact Initiative BW.mn.BRC10269). The authors acknowledge the facilities and scientific and technical assistance of the National Imaging Facility, a National Collaborative Research Infrastructure Strategy (NCRIS) capability, at the Center for Microscopy, Characterization, and Analysis, the University of Western Australia. This research was funded in whole, or in part, by the Wellcome Trust [WT 218380]. For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Materials
| Name | Company | Catalog Number | Comments |
| Brainlab Neuronavigation System | Brianlab, Westchester, IL | https://www.brainlab.com/surgery-products/overview-neurosurgery-products/cranial-navigation/ | Intraoperative neuronavigation system |
| EpiNav Planning Software | N/A | N/A | Clinical Decision Support Tool, for research use, developed in academia at King's College London and University College London |
| Mayfield clamp | Integra | A1059 | Any 3 pin head immobilisation device can be used |
| Microsurgical instruments | As per local neurosurgical unit | ||
| MRI Scanner | GE, Milwaukee, WI, USA | 3T MRI GE MR750 | Any alternative 3T MRI scanner can be used |
| MRTrix3 | N/A | Reference 47 in the manuscript | MRtrix3 provides a set of tools to perform various advanced diffusion MRI analyses, including constrained spherical deconvolution (CSD), probabilistic tractography, track-density imaging, and apparent fibre density |
| NORAS coil | NORAS MRI Products | https://www.noras.de/en/mri-produkte/lucy-or-head-holder-8-ch-coil/#infos | Any MRI-safe head immobilisation device can be used |
| Perforator drill | Stryker | https://neurosurgical.stryker.com/products/elite/ | Any alternative neurosurgical perforator drill driver and bit can be used |
| Sutures - Vicryl Plus 2/- | Ethicon | ETVCP684H | Any alternative suture that the surgeon prefers can be used |
| Titanium bone plates and screws | As per local neurosurgical unit | ||
| Ultrasonic Aspirator | Integra | https://products.integralife.com/cusa-tissue-ablation/category/cusa-tissue-ablation | Any alternative that the surgeon prefers can be used |
References
- Marathe, K., et al. ablative and radiosurgical interventions for drug-resistant mesial temporal lobe epilepsy: A systematic review and meta-analysis of outcomes. Front Neurol. 12, 777845 (2021).
- Choi, H., et al. Epilepsy surgery for pharmacoresistant temporal lobe epilepsy: A decision analysis. JAMA. 300, 2497 (2008).
- Foldvary, N., et al. Seizure outcome after temporal lobectomy for temporal lobe epilepsy: A Kaplan-Meier survival analysis. Neurology. 54, 630-634 (2000).
- Spencer, S. S., et al. Predicting long-term seizure outcome after resective epilepsy surgery: The multicenter study. Neurology. 65, 912-918 (2005).
- Sperling, M. R., O'Connor, M. J., Saykin, A. J., Plummer, C. Temporal lobectomy for refractory epilepsy. JAMA. 276, 470-475 (1996).
- Wiebe, S. Effectiveness and safety of epilepsy surgery: What is the evidence. CNS Spectr. 9, 120-132 (2004).
- Gilliam, F., et al. Patient-oriented outcome assessment after temporal lobectomy for refractory epilepsy. Neurology. 53, 687-694 (1999).
- Markand, O. N., Salanova, V., Whelihan, E., Emsley, C. L. Health-related quality of life outcome in medically refractory epilepsy treated with anterior temporal lobectomy. Epilepsia. 41, 749-759 (2000).
- Jones, J. E., Berven, N. L., Ramirez, L., Woodard, A., Hermann, B. P. Long-term psychosocial outcomes of anterior temporal lobectomy. Epilepsia. 43, 896-903 (2002).
- Spencer, D. D., Spencer, S. S., Mattson, R. H., Williamson, P. D., Novelly, R. A. Access to the posterior medial temporal lobe structures in the surgical treatment of temporal lobe epilepsy. Neurosurgery. 15, 667-671 (1984).
- Vakharia, V. N., et al. Intraoperative overlay of optic radiation tractography during anteromesial temporal resection: A prospective validation study. J Neurosurg. 1, 1-10 (2021).
- Winston, G. P., et al. Preventing visual field deficits from neurosurgery. Neurology. 83, 604-611 (2014).
- Almairac, F., Herbet, G., Moritz-Gasser, S., de Champfleur, N. M., Duffau, H. The left inferior fronto-occipital fasciculus subserves language semantics: A multilevel lesion study. Brain Struct Funct. 220, 1983-1995 (2015).
- Binding, L. P., Dasgupta, D., Giampiccolo, D., Duncan, J. S., Vos, S. B. Structure and function of language networks in temporal lobe epilepsy. Epilepsia. 63, 1025-1040 (2022).
- Giampiccolo, D., Duffau, H. Controversy over the temporal cortical terminations of the left arcuate fasciculus: A reappraisal. Brain. 145, 1242-1256 (2022).
- Sone, D., et al. Optimal surgical extent for memory and seizure outcome in temporal lobe epilepsy. Ann Neurol. 91, 131-144 (2022).
- Galovic, M., et al. Association of piriform cortex resection with surgical outcomes in patients with temporal lobe epilepsy. JAMA Neurol. 76, 690-700 (2019).
- Dasgupta, D., et al. Hippocampal resection in temporal lobe epilepsy: Do we need to resect the tail. Epilepsy Res. 190, 107086 (2023).
- Wu, C., et al. Effects of surgical targeting in laser interstitial thermal therapy for mesial temporal lobe epilepsy: A multicenter study of 234 patients. Epilepsia. 60, 1171-1183 (2019).
- Wiebe, S. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 345 (5), 311-318 (2001).
- Brotis, A. G., et al. A meta-analysis on potential modifiers of LITT efficacy for mesial temporal lobe epilepsy: Seizure-freedom seems to fade with time. Clin Neurol Neurosurg. 205, 106644 (2021).
- Borger, V., et al. Resection of piriform cortex predicts seizure freedom in temporal lobe epilepsy. Ann Clin Transl Neurol. 8, 177-189 (2020).
- Borger, V., et al. Temporal lobe epilepsy surgery: Piriform cortex resection impacts seizure control in the long-term. Ann Clin Transl Neurol. 9 (8), 1206-1211 (2022).
- Hwang, B. Y., et al. Piriform cortex ablation volume is associated with seizure outcome in mesial temporal lobe epilepsy. Neurosurgery. 91, 414-421 (2022).
- Piper, R. J., et al. Extent of piriform cortex resection in children with temporal lobe epilepsy. Ann Clin Transl Neurol. 10, 1613-1622 (2023).
- Allison, A. C. The secondary olfactory areas in the human brain. J Anat. 88, 481-488 (1954).
- Ribas, G. C. The cerebral sulci and gyri. Neurosurg Focus. 28, E2 (2010).
- Young, J. C., Vaughan, D. N., Paolini, A. G., Jackson, G. D. Electrical stimulation of the piriform cortex for the treatment of epilepsy: A review of the supporting evidence. Epilepsy Behav. 88, 152-161 (2018).
- Mai, J. K., Majtanik, M., Paxinos, G. . Atlas of the Human Brain. , (2016).
- Vaughan, D. N., Jackson, G. D. The piriform cortex and human focal epilepsy. Front Neurol. 5, 259 (2014).
- Gale, K. Progression and generalization of seizure discharge: Anatomical and neurochemical substrates. Epilepsia. 29, S15-S34 (1988).
- Löscher, W., Ebert, U. The role of the piriform cortex in kindling. Prog Neurobiol. 50, 427-481 (1996).
- Piredda, S., Gale, K. A crucial epileptogenic site in the deep prepiriform cortex. Nature. 317, 623-625 (1985).
- McIntyre, D. C., Gilby, K. L. Mapping seizure pathways in the temporal lobe. Epilepsia. 49, 23-30 (2008).
- Fahoum, F., Lopes, R., Pittau, F., Dubeau, F., Gotman, J. Widespread epileptic networks in focal epilepsies: EEG-fMRI study. Epilepsia. 53, 1618-1627 (2012).
- Flanagan, D., Badawy, R. A. B., Jackson, G. D. EEG-fMRI in focal epilepsy: Local activation and regional networks. Clin Neurophysiol. 125, 21-31 (2014).
- Laufs, H., et al. Converging PET and fMRI evidence for a common area involved in human focal epilepsies. Neurology. 77, 904-910 (2011).
- Wieser, H. G., et al. Proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia. 42, 282-286 (2001).
- Dasgupta, D., Duncan, J. S. Optimizing epilepsy surgery. ISRCTN Registry. BMC. , (2020).
- Sparks, R., et al. Automated multiple trajectory planning algorithm for the placement of stereo-electroencephalography (SEEG) electrodes in epilepsy treatment. Int J CARS. 12, 123-136 (2017).
- Dasgupta, D. . Improving outcomes in anteromesial temporal lobe resections - A prospective surgical trial integrating multimodal imaging & novel hi-res tractography. , (2022).
- Winston, G. P., et al. Automated T2 relaxometry of the hippocampus for temporal lobe epilepsy. Epilepsia. 58, 1645-1652 (2017).
- Cardoso, M. J., et al. Geodesic information flows: Spatially-variant graphs and their application to segmentation and fusion. IEEE Trans Med Imaging. 34, 1976-1988 (2015).
- Winston, G. P., et al. Automated hippocampal segmentation in patients with epilepsy: Available free online. Epilepsia. 54, 2166-2173 (2013).
- Iqbal, S., et al. Volumetric analysis of the piriform cortex in temporal lobe epilepsy. Epilepsy Res. 185, 106971 (2022).
- Leon-Rojas, J. E., et al. Resection of the piriform cortex for temporal lobe epilepsy: a novel approach on imaging segmentation and surgical application. Br J Neurosurg. 1, 1-6 (2021).
- Tournier, J. -. D., et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualization. Neuroimage. 202, 116137 (2019).
- Cordero-Grande, L., Christiaens, D., Hutter, J., Price, A. N., Hajnal, J. V. Complex diffusion-weighted image estimation via matrix recovery under general noise models. Neuroimage. 200, 391-404 (2019).
- Vos, S. B., et al. The importance of correcting for signal drift in diffusion MRI. Magn Reson Med. 77, 285-299 (2017).
- Kellner, E., Dhital, B., Kiselev, V. G., Reisert, M. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn Reson Med. 76, 1574-1581 (2016).
- Smith, S. M., et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 23, S208-S219 (2004).
- Andersson, J. L. R., Sotiropoulos, S. N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 125, 1063-1078 (2016).
- Leemans, A., Jones, D. K. The B-matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med. 61, 1336-1349 (2009).
- Tustison, N. J., et al. N4ITK: Improved N3 Bias Correction. IEEE Trans Med Imaging. 29, 1310-1320 (2010).
- Dhollander, T., Raffelt, D., Connelly, A. Unsupervised 3-tissue response function estimation from single-shell or multi-shell diffusion MR data without a co-registered T1 image. ISMRM Workshop on Breaking the Barriers of Diffusion MRI. 5, (2016).
- Binding, L. P., et al. Contribution of white matter fiber bundle damage to language change after surgery for temporal lobe epilepsy. Neurology. 100, e1621-e1633 (2023).
- Giampiccolo, D., et al. Thalamostriatal disconnection underpins long-term seizure freedom in frontal lobe epilepsy surgery. Brain. 146, 2377-2388 (2023).
- Smith, R. E., Tournier, J. -. D., Calamante, F., Connelly, A. Anatomically-constrained tractography: Improved diffusion MRI streamlines tractography through effective use of anatomical information. Neuroimage. 62, 1924-1938 (2012).
- Tournier, J. D., Calamante, F., Connelly, A. Improved probabilistic streamlines tractography by 2nd order integration over fibre orientation distributions. Proc 18th Annu Meet ISMRM. 1670, (2010).
- Yasargil, M. G., Fox, J. L. The microsurgical approach to intracranial aneurysms. Surg Neurol. 3, 7-14 (1975).
- Yasargil, M. G., et al. Microsurgical pterional approach to aneurysms of the basilar bifurcation. Surg. Neurol. 6, 83-91 (1976).
- Rao, D., Le, R. T., Fiester, P., Patel, J., Rahmathulla, G. An illustrative review of common modern craniotomies. J Clin Imaging Sci. 10, 81 (2020).
- Usui, N., Kondo, A., Nitta, N., Tottori, T., Inoue, Y. Surgical resection of amygdala and uncus. Neurol Med Chir (Tokyo). 58, 377-383 (2018).
- Vivas, A. C., Reintjes, S., Shimony, N., Vale, F. L. Surgery of the amygdala and uncus: A case series of glioneuronal tumors. Acta Neurochir. (Wien). 162, 795-801 (2020).
- Al-Otaibi, F., Baeesa, S. S., Parrent, A. G., Girvin, J. P., Steven, D. Surgical techniques for the treatment of temporal lobe epilepsy. Epilepsy Res Treat. 2012, 1-13 (2012).
- Galovic, M., et al. Association of piriform cortex resection with surgical outcomes in patients with temporal lobe epilepsy. JAMA Neurol. 76, 690 (2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved