Method Article
Measuring In Vivo Adipose Tissue Kinetics in Humans Using the Deuterium (2H)-Labeling Approach
In This Article
Summary
Presented here is a protocol to measure in vivo adipose tissue kinetics in humans using the deuterium (2H)-labeling method.
Abstract
White adipose tissue is a highly plastic organ that is necessary to maintain whole-body energy homeostasis. The adipose tissue mass and changes in the fat mass or distribution are regulated by changes in the synthesis and breakdown (i.e., turnover) of adipose cells and triacylglycerols. Evidence suggests that the manner and magnitude of subcutaneous adipose tissue expansion (i.e., hypertrophy vs. hyperplasia) and turnover can influence metabolic health, as adipogenesis has been implicated in the pathogenesis of obesity and related diseases. Despite the potential role of adipose turnover in human health, there is a lack of knowledge about the in vivo kinetics of adipose cells. This is due, in part, to the slow turnover rate of the cells in adipose tissue and the practical complexity of directly labeling their metabolic precursors in vivo. Herein, we describe methods to measure in vivo adipose kinetics and turnover rates in humans through the consumption of deuterium (2H)-labeled water. The incorporation of 2H into the deoxyribonucleotide moieties of DNA in pre-adipocytes and adipocytes provides an accurate measure of cell formation and death (adipose turnover). Overall, this is an innovative approach to measuring in vivo adipose kinetics and represents a substantive departure from other in vitro assessments.
Introduction
Obesity is a disease characterized by excess white adipose tissue (AT) and is a significant risk factor for the development of Type II diabetes and cardiovascular disease1. White AT is a highly plastic organ that stores energy in the form of triacylglycerols (TGs) and is essential for metabolic homeostasis2. White AT retains the ability to expand, reduce, and remodel during adulthood3, and the AT mass is determined by dynamic changes in the adipocyte volume (via TG synthesis and breakdown), continual adipocyte formation via the proliferation and differentiation of pre-adipocytes (i.e., hyperplasia or adipogenesis), and adipose cell death4.
Evidence suggests that there is an important link between the subcutaneous AT turnover (e.g., adipocyte formation and death) and cardiometabolic health5,6,7,8, and the role of adipogenesis in the pathogenesis of obesity-related disorders remains debatable4. However, little is known about in vivo AT turnover in humans due, in part, to the slow turnover rate of the cellular components of the AT and the complexity of directly labeling their metabolic precursors in vivo. While in vitro methods have provided some insight, these approaches do not provide a comprehensive in vivo assessment within the natural milieu of the AT.
A method was developed by the Hellerstein laboratory9 to assess in vivo AT turnover using the incorporation of the stable isotope deuterium (2H) from heavy water (2H2O) into the AT (Figure 1)10. The protocol, which has been validated in mice and humans, includes an initial ramp-up of 2H2O to increase the 2H enrichment of the body water, followed by adequate daily intake to maintain stable, near-plateau enrichment values. The 2H from the 2H2O (i.e., heavy water) is incorporated into the deoxyribose (dR) moiety of deoxyribonucleotides in the DNA of adipose cells, and the isotope enrichment is measured in the DNA via mass spectrometry and the application of mass isotopomer distribution analysis (MIDA)9,10,11. Labeling the deoxyribose moiety of purine deoxyribonucleotides in DNA with stable isotope precursors has several advantages over previous methods, such as those that involved labeling with pyrimidine nucleotide base moieties (e.g., from 3H-thymidine or bromo-deoxyuridine). Of note, the endogenous reincorporation of bases, especially for pyrimidines, but not dR, in replicating DNA previously confounded the interpretation of label incorporation12. In addition, the incorporation of a stable isotope label into dR causes no genotoxicity, in contrast to the incorporation of radioactive or genotoxic agents such as 3H (tritium) or bromo-deoxyuridine. Therefore, the long-term use of this technique in animal models and humans is safe.
The measurement of 2H-labeled DNA synthesis denotes the passage of a cell through the S-phase of cell division and identifies newly formed pre-adipocytes and adipocytes (via pre-adipocyte differentiation) or adipogenesis13. Cells that undergo rapid turnover (e.g., monocytes) replace their DNA quickly and reach a plateau in 2H-enrichment, thus providing an internal reference for the assay. The ratio of the 2H-enrichment of DNA from adipose cells to that of monocytes (reference cells) or the integrated body 2H2O measurement allows the calculation of the fraction of newly synthesized adipose cells. Herein, this protocol describes methods to measure in vivo adipose cell turnover (adipogenesis) rates in humans via the 2H metabolic labeling protocol, including refined techniques to purify the adipocytes via negative immune selection and to enrich the pre-adipocyte population14.
Protocol
Pennington Biomedical Research Center's Institutional Review Board (IRB) approved all the procedures (#10039-PBRC), and all human subjects gave written informed consent.
1. Eight week 2H2O-labeling period
- Administer aliquots of either 70% or 99.9% deuterium-labeled water (2H2O) in sterile plastic containers.
- Instruct the participants to drink 35 mL doses of 99.9% enriched 2H2O or 40 mL doses of 70% enriched 2H2O three times per day in week 1 (priming period) and to drink two 35 mL or 50 mL doses, respectively, per day in weeks 2-8.
NOTE: Transient dizziness or vertigo are the only known adverse effects of 2H2O intake and are related to rapid changes in the bulk water flow, which are perceived by the hair follicles of the inner ear. Hence, instruct the participants to take the doses at least ~2 h apart to avoid the rare occurrence of transient dizziness or vertigo15. For the same reason, do not make up for missed doses by doubling a single dose. Administering the dose as described above (separated by ≥2 h) makes this adverse effect extremely rare in human subjects (<1% of several hundred subjects). - Monitor the compliance with 2H2O intake through urine collections to measure the 2H enrichment in the body water and also by the weekly return of the empty vials for counting.
- Clean the urine samples using activated charcoal and a filter.
- Add 8 mL of urine to a 10 mL tube with 1 mL of activated charcoal.
- Place the sample on a rocker for 10 min, and centrifuge for 5 min at 800 x g so that the charcoal moves to the bottom of the tube. Filter the sample with a 0.2 µm syringe filter.
- Measure the 2H2O enrichment in the body water (urine) by isotope ratio mass spectrometry (IRMS).
NOTE: 2H2O enrichment can be analyzed by different methods, including gas chromatography-mass spectrometry16, high-temperature conversion elemental analyzer (TC/EA) coupled with IRMS17, cavity ring-down infrared spectroscopy (IRIS)18, or with an H/Device attached to a mass spectrometer (e.g., IRMS)19. - Use the mean 2H2O enrichments measured in the urine during the 8 week labeling period to calculate the precursor 2H2O exposure and the fraction of newly synthesized AT cells (see section 7 and section 8 below).
- Clean the urine samples using activated charcoal and a filter.
NOTE: The 2H2O labeling protocol maintains near-plateau 2H enrichment in the body water within the range of 1.0%-2.5% for the duration of the 8 week labeling period (Figure 2)10.
2. Adipose tissue biopsy collections from human subjects
- After cleansing the skin with povidone-iodine solution, administer topical anesthesia (e.g., 2% lidocaine/0.5% bupivacaine), make a ~0.75 cm incision in the skin, and collect subcutaneous AT biopsies via the needle lipoaspiration technique under sterile conditions13.
- Weigh a sterile 50 mL tube containing 5 mL of room temperature (RT) 1 M HEPES buffer, pH 7.3. Immediately place the AT in the tube with the HEPES buffer for processing.
- Weigh the 50 mL tube containing the AT, and record the weight.
3. Isolation of purified adipocytes
- Add a type 1 collagenase /HEPES (2 mg/mL) solution to 2 g/mL AT.
- Digest the AT by incubating in a water bath with shaking (100 rpm) for 1 h at 37 °C until there is a homogenous mixture with a few large, intact pieces of AT.
- Centrifuge the tube at 500 x g for 8 min at RT to separate the adipocytes and the stromal-vascular fraction (SVF).
- Gently remove the top fat layer (adipocytes), and transfer it to a separate tube. Do not disrupt the pellet (SVF) at the bottom of the tube.
- As previously described14, purify the adipocytes using immunomagnetic cell separation.
NOTE: This important step is done to "clean" the adipocytes, as other cell types with fast cell turnover, including hematopoietic, endothelial, and stem cells, may adhere to the floating adipocytes and impact the measurements.- Place ~400 µL of adipocytes into a 5 mL tube for negative immuno-purification.
- Add FcR blocking antibody (100 µL/mL).
- Add a cocktail of biotinylated antibodies against markers of endothelial cells (anti-human CD31; 1:100), hematopoietic cells (anti-human CD45; 1:400), and mesenchymal stem cells (anti-human CD34; 1:100) to the adipocyte solution for 15 min at RT.
- Add a biotin selection cocktail (100 µL/mL) to the adipocyte solution, mix well, and incubate for 15 min at RT with gentle tilting/rotation.
- Mix the magnetic nanoparticles by pipetting up and down, add the particles (50 µL/mL) to the adipocyte solution, mix well, and incubate for 10 min at RT with gentle rotation (10 rpm).
- Add PBS/2% FBS/1 mM EDTA buffer to the adipocyte solution for a total volume of 1 mL.
- Place the tube in the magnet, and let it sit for 5 min.
- Pick up the magnet, and in one continuous motion, invert the magnet containing the tube, and pour the contents into a new 5 mL tube. Do not tap the tube while pouring. The cells attached to the antibodies are bound by the magnetic nanoparticles and removed, while the immuno-purified adipocytes are retained.
- Flash-freeze the purified adipocytes in liquid N2, and store them at −80 °C until the DNA extraction.
4. Isolation of pre-adipocytes
- To isolate an enriched population of pre-adipocytes, employ a protocol to exploit their ability to attach to plastic after a short-term culture of the SVF20.
- In a laminar flow hood, resuspend the SVF pellet in 5 mL of erythrocyte lysis buffer for 5-10 min at RT (mix thoroughly), and centrifuge at 800 x g. Remove the supernatant, and resuspend the pellet in 10% FBS in alpha (α)MEM.
- Plate the cells on a plastic culture dish for ~8-12 h in a tissue culture incubator at 37 °C and with a 5% CO2 atmosphere.
- After ~8-12 h, gently wash the non-adherent cells from the culture plate with PBS inside a laminar flow hood.
- After aspirating the PBS, add 1-1.5 mL of 0.25% trypsin/1 mM EDTA to the culture dish to detach the adherent cells (enriched population of pre-adipocytes). Place the culture dish in the incubator at 37 °C for ~5-8 min to help lift the cells.
- Add 10% FBS/αMEM to the plate, and wash the plate thoroughly to collect all the cells from the plate. Transfer the cell solution into a 15 mL or 50 mL tube, and centrifuge at 800 x g for 8 min.
- Remove the supernatant, and store the pellet (pre-adipocytes) at −80 °C until the DNA extraction.
5. Isolation of blood monocytes
NOTE: The monocytes are analyzed to represent a (nearly) completely turned-over cell population, and the measurement of the 2H enrichment in the monocytes can be used as a reference marker of 2H2O exposure in each individual. Alternatively, the body 2H2O enrichment can be measured and used to calculate the 2H2O exposure.
- Draw fresh, whole blood from human subjects into vacutainer tubes containing EDTA.
- Centrifuge the blood at 1,000-2,000 x g for 15 min at 4 °C.
NOTE: Do not use the brake. - Remove the top layer of plasma. Be careful not to touch the white buffy coat just under the plasma.
- Aspirate the white buffy coat using a transfer pipette, and transfer it to a 50 mL tube. Add ~10 mL of PBS to the buffy coat.
- Gently add 10 mL of a density gradient medium to layer the buffy coat.
NOTE: Make sure the tip of the pipette touches the bottom of the tube, and eject carefully so that there will be a clear layer of density gradient medium at the bottom. Avoid introducing bubbles. - Centrifuge the tube at 800 x g for 30 min with the brake off.
- Pipette out (~10 mL) the white layer (mononuclear fraction), making circular movements with the tip of the transfer pipette close to the border but not touching the upper layer. Transfer to a new 50 mL tube, and add 10 mL of PBS. Centrifuge at 800 x g for 5 min (do not use the brake), and discard the supernatant.
- Add 5 mL of erythrocyte lysis buffer to the pellet. Mix, and let it sit for 2 min at RT.
- Add 5 mL of PBS with 0.1% BSA to the erythrocyte lysis buffer/pellet solution, centrifuge at 800 x g for 5 min, and discard the supernatant.
- Isolate the monocytes as CD14+ cells using immuno-magnetic beads. Perform the isolation of the CD14+ cells by following the manufacturer's protocol.
- Store the isolated monocytes at −80 °C until the DNA extraction.
6. DNA preparation (isolation, hydrolysis, and derivatization)
- Isolate the DNA from the pre-adipocytes, adipocytes, and blood monocytes using a DNA extraction kit following the manufacturer's instructions.
- To free the deoxyribonucleosides, enzymatically hydrolyze the DNA (~200 µL) overnight (no more than 24 h) at 37 °C in 50 µL of enzyme hydrolysis cocktail containing 1 mL of S1 nuclease, 1 mL of phosphatase enzyme, and 36.8 mL of 5x hydrolysis buffer in 16 mm x 100 mm (10 mL) screw-capped glass tubes. Additionally, include the following samples: a water blank, a hydrolysis cocktail blank, a column blank from the DNA extraction kit, and 500 ng of DNA standards.
- To prepare the 5x hydrolysis buffer, place 94 mL of molecular biology-grade pure water into a sterile beaker. Weigh out 3.08 g of sodium acetate and 21.5 mg of zinc sulfate, add water, and mix until dissolved. Adjust the pH to 5.0 with glacial acetic acid, and test using pH paper. Add water to reach a total volume of 100 mL.
- To prepare the phosphatase enzyme, resuspend a vial of acid phosphatase in 1 mL of pure water.
- To prepare the 0.5 U/µL S1 nuclease enzyme, dilute 2.5 µL of S1 nuclease into 2 mL of 1x hydrolysis buffer (add 1 mL of 5x hydrolysis buffer to 4 mL of pure water).
- Derivatize the hydrolysates to pentafluorobenzylhydroxylamine (PFBHA) derivatives. Specifically, add the following directly to the digested DNA samples, including the standards and blanks, in 16 mm x 100 mm (10 mL) screw cap tubes: 100 µL of pentafluorobenzyl hydroxylamine hydrochloride (PFBHA; 1 mg/mL) and 75 µL of glacial acetic acid. Vortex (briefly), cap the vials, and place the hydrolysate samples on a heating block set at 100 °C for 30 min.
- Remove the samples, and let them cool for ~5 min to RT. After cooling, add 2 mL of acetic anhydride and 100 µL of 1-methylimidazole to each tube under a fume hood. Set the tubes on a 100 °C heat block for 5 min.
- Remove the samples, and then allow them to cool for ~15-20 min. Once cool, add 3 mL of molecular biology-grade water to each sample, vortex briefly, and let them sit for 10 min.
- Add 2 mL of dichloromethane (DCM) to the tubes, and vortex the samples vigorously for 15 s to extract the derivative into the organic phase.
- Centrifuge at 800 x g for 5 min to separate the phases. Carefully transfer the bottom dichloromethane layer into a clean 1.6 mL GC vial.
NOTE: Do not transfer any of the aqueous phase, as this will increase the background. - Evaporate to dryness with nitrogen for ~20 min to remove the DCM and residual acetic acid, followed by a ~10 min dry in a speed vacuum at RT to remove all traces of the reagents.
7. Gas chromatography-mass spectrometry (GC-MS) analysis of the DNA
- Once dry, resuspend the PFBHA derivatives in 150 µL of ethyl acetate, and cap. Next, analyze for the incorporation of 2H into the DNA on a gas chromatography (GC)/mass spectrometry (MS) instrument equipped with a DB-225 column using methane negative chemical ionization and collecting the ions in selective ion-monitoring mode at m/z 435, m/z 436, and m/z 437 (representing the M0, M1, and M2 mass isotopomers, respectively). Follow the manufacturer's instructions for the instrument used.
- Set the GC conditions as follows: column: 30 m x 0.25 mm ID x 0.25 µm film coating; chromatograph: helium carrier gas at 1.0 mL/min constant flow; pulsed split-less injections; injector temperature: 250 °C; MS transfer line: 325 °C; oven conditions: initial temperature 100 °C, hold 2 min, ramp at 40 °C/min to a temperature of 220 °C, hold 7.5 min, second ramp at 40 °C/min to 320 °C, hold for 1 min. The total runtime is 16.0 min. The typical dR elution times are 10.5 min and 10.8 min for the first and second peaks, respectively.
- Set the MS conditions as follows: methane negative chemical ionization source with detection in single ion monitoring mode; ions collected for dR: m/z 435, m/z 436, and m/z 437 (representing M0, M1, and M2 mass isotopomers, respectively).
NOTE: Siliconize the open glass liner with a small plug of siliconized glass wool in the lower portion of the liner. Change this liner often (every ~100 injections).
- Calculate the mass isotopomer abundance M1 ratios in the unenriched (natural abundance) dR derivative as follows:
M1 ratio = M1 abundance/(sum of M0, M1, and M2 abundances)
Then, subtract the natural abundance M1 ratios from the M1 ratios in the dR of the labeled samples to calculate the enrichments (excess isotope abundances, or %EM1)21. - Measure the mass isotopomer abundances of the baseline (unenriched) DNA standards concurrently over a range of M0 ion abundances that span the range of M0 ion abundances in the samples analyzed.
NOTE: This step is essential to correct for the "abundance sensitivity" of isotope ratios measured by GC/MS22. - Construct a graph of the measured M0 ion abundance versus the measured mass isotopomer M1 ratios in the unlabeled standards to determine the on-column sample concentration that is most accurate for the M1 mass isotopomer (i.e., where it is closest to the known, calculated natural abundance M1 ratio, or the "sweet spot"). The M1 ratio for this unenriched dR derivative is 0.1669.
- Inject the experimental samples so that they are as close as possible in the M0 peak area to this "sweet spot". Use a quadratic regression of the M1 ratio of the unenriched standards versus the M0 peak area to estimate an "adjusted natural abundance" M1 ratio for each sample at its particular M0 concentration. Subtract this from the adipose cell sample M1 ratio to yield the percent enrichment above the natural abundance, or %EM1 (Figure 3)23.
- Calculate the theoretical maximum M1 enrichment (EM1*) in the adipose cells using mass isotopomer distribution analysis (MIDA) equations24 based on the body 2H2O exposure (measured in the urine) integrated over the 8 week period (Figure 4) or the interim measured monocyte enrichments (step 7.5).
8. Calculation of the fraction of newly synthesized cells, or in vivo adipogenesis
- Calculate the fraction of new cells (%), which is a measure of the in vivo adipogenesis or the formation of newly synthesized pre-adipocytes or adipocytes, using the following formula:
Fraction of new cells (%) = [(M1 enrichment in the sample [adipose] cells)/EM1* (theoretical maximum M1 enrichment)] x 100
NOTE: *The 2H enrichment in monocytes can also be used as the denominator of the equation. This measurement in monocytes represents a reference cell marker of the 2H2O exposure in each individual and may be used to confirm the calculations of theoretical maximum M1enrichment from the measured body 2H2O values.
Results
The 2H2O labeling protocol (section 1) maintains near-plateau 2H enrichment in the body water within the range of 1.0%-2.5% for the duration of the 8 week labeling period10, as shown in Figure 2. A previous study utilized the 2H2O labeling protocol to assess adipose kinetics via the incorporation of 2H into the DNA of adipose cells, as detailed in sections 2-8, and reported that in vivo adipogenesis (both pre-adipocyte proliferation and adipocyte formation) was higher in the subcutaneous femoral (scFEM) relative to the subcutaneous abdominal (scABD) depot in women with obesity (Figure 5)13. Additional data showed that pre-adipocyte and adipocyte formation rates in the subcutaneous AT depots were positively associated with the overall percentage body fat (Figure 6)13. Overall, these published data confirm that this physiologic 2H labeling method is an innovative approach for assessing in vivo AT kinetics in individuals with varying adipose distributions and body fat contents13.
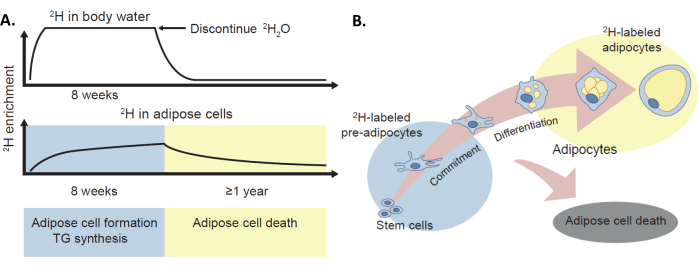
Figure 1: The 2H labeling protocol to assess in vivo adipose turnover. (A) The deuterium labeling method has been validated to provide in vivo estimates of adipose cell formation, TG synthesis, and adipocyte death in humans via the incorporation of the stable isotope 2H, administered as 2H2O, into the body water and adipose tissue. (B) The enrichment of 2H in the adipose tissue (pre-adipocytes and adipocytes) provides a measure of adipose cell formation. Of note, this protocol can also provide a measure of adipose cell death via 2H label loss, although this is not the primary topic of this manuscript. This figure has been adapted with permission from White and Ravussin4. Please click here to view a larger version of this figure.
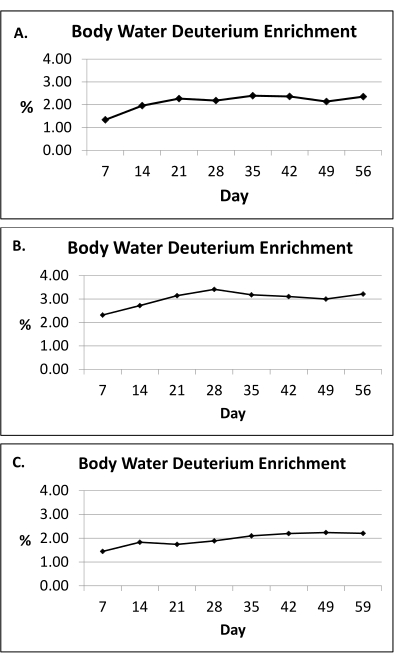
Figure 2: Body water deuterium enrichment. (A-C) Presented here are representative body water deuterium enrichment measurements (urine) during the 8 week 2H2O labeling protocol in three human subjects. Based on the doses provided (section 1), the 2H2O labeling protocol maintains near-plateau 2H enrichment in the body water within the range of 1.0%-2.5% (y-axis) for the duration of the 8 week labeling period (x-axis). The average body water enrichment is calculated using an area-under-the-curve approach for the entire exposure period. Please click here to view a larger version of this figure.

Figure 3: Natural abundance correction curve of unlabeled DNA. The analysis of unlabeled DNA by mass spectrometry results in a rising baseline M1 fractional abundance, falsely suggesting increasing enrichment with increasing sample load on the GC column. An unlabeled abundance curve is necessary to correct this "abundance sensitivity" of the isotope ratios. This is done by using a range of unlabeled DNA standard loads (M0 peak areas) and subtracting the measured M1 fractional abundance (M1 ratio = M1 abundance/abundance of M0, M1, and M2) that corresponds to the M0 peak area of a labeled sample. In the example shown, the two pairs of 1.35% enriched quality control samples only yield correct results after subtracting each appropriate M1 fractional abundance value obtained from the unlabeled curve. The calculated M1 ratio for the dR derivative is 0.1669. In this figure, the M1 ratio is reached at 82 million M0 peak area counts. In this example, an "adjusted" M1 ratio for unenriched dR can be calculated using the following regression: y = 1.3024e−19x2 + 1.7010e−11x + 0.1650. This figure has been adapted with permission from Mazzarello et al.23 Please click here to view a larger version of this figure.

Figure 4: Relationship between body water enrichment (p) and the maximum enrichment of a fully turned-over tissue. Mass isotopomer distribution tables are constructed, which calculate all the isotopomers of dR at all possible body water enrichments (p). These are then used to derive the relationship between p and the maximum M1 enrichment (EM1*) of dR in newly replicated DNA. If the body water enrichment is known, EM1* can be calculated and used to calculate the fractional synthesis. This is useful when a fully turned-over reference tissue is unavailable. The regressions of Busch et al.9 and Mazzarello et al.23 produce comparable fractional synthesis results as long as all the measurements consistently use either two masses or three masses in the calculation of the M1 ratios and EM1*. Please click here to view a larger version of this figure.
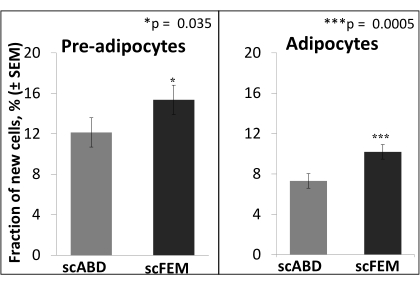
Figure 5: Higher formation rates of pre-adipocytes and adipocytes in the scFEM depot relative to the scABD depot. The least-square means comparing the fraction of new pre-adipocytes and adipocytes in different depots were derived from the linear mixed model (n = 25). The difference in the fraction of new pre-adipocytes between the subcutaneous femoral (scFEM) and subcutaneous abdominal (scABD) depots was 3.224 (p = 0.0354), while the difference in the fraction of new adipocytes between the scFEM and scABD depots was 2.877 (p = 0.0005)13. The study population included 25 women of African-American (n = 14) and Caucasian (n = 11) ancestry, with a mean age of 31 years ± 6 years, a mean BMI of 32.6 kg/m2 ± 2.7 kg/m2, and 44.3% ± 4.1% body fat. This figure has been adapted with permission from White et al.13. Please click here to view a larger version of this figure.

Figure 6: Positive correlation between pre-adipocyte and adipocyte formation in both scABD and scFEM (C,D) depots and the overall percentage body fat. Simple associations between the fraction of new pre-adipocytes or adipocytes and the percentage body fat were analyzed using Spearman's correlation (n = 25). (A,B) The Spearman's correlation between the fraction of new pre-adipocytes (subcutaneous abdominal; scABD) and the percentage body fat was 0.4263 (R2 = 0.2472; p = 0.019), and the Spearman's correlation between the fraction of new adipocytes (scABD) and the percentage body fat was 0.3291 (R2 = 0.2346; p = 0.026). (C,D) The Spearman's correlation between the fraction of new pre-adipocytes (subcutaneous femoral; scFEM) and the percentage body fat was 0.2761 (R2 = 0.1123; p = 0.092), and the Spearman's correlation between the fraction of new adipocytes (scFEM) and the percentage body fat was 0.5358 (R2 = 0.2116; p = 0.056)13. The study population included 25 women of African-American (n = 14) and Caucasian (n = 11) ancestry, with a mean age of 31 years ± 6 years, a mean BMI of 32.6 kg/m2 ± 2.7 kg/m2, and 44.3% ± 4.1% body fat. This figure has been adapted with permission from White et al.13. Please click here to view a larger version of this figure.
Discussion
In vivo assessments are necessary to provide new knowledge on the dynamics of white AT turnover and its role in obesity and related metabolic diseases, as in vitro assessments do not encompass the natural environment of the AT. Although the use of retrospective radiocarbon dating to assess adipose dynamics has been informative7,25, this approach is not suitable for capturing dynamic changes during prospective intervention studies. The 2H labeling method has been validated to provide physiological measures of in vivo adipose cell formation and turnover in humans and represents a substantive departure from other assessments.In recent years, this approach has been applied to assess and uncover novel adipose kinetics in both rodents26 and humans13,27,28.
Notably, with this method, additional AT processing techniques were implemented to purify the freshly isolated adipocytes and remove contamination from other cell types that may adhere to the floating adipocytes and impact the measurements. As previously reported, these steps resulted in a favorable improvement in the accuracy of the in vivo adipogenesis measurements, specifically the measurement of newly formed adipocytes14. Another additional step in this method was performing a short-term culture of SVF cells to allow the efficient and preferential attachment of adipocyte progenitor cells. This culture step was found to enrich the population of pre-adipocytes, resulting in more accurate measures of in vivo proliferation14. The purity of the cells of interest is an important consideration in this method, especially when analyzing tissues comprising various cell types.
While adipocyte death is a significant component of AT turnover8, an assessment of cell death was not included in the protocol, and, thus, adipocyte death was not measured or discussed in this analysis. However, the fractional synthesis rate (FSR), or the fraction of newly formed cells present in the AT over the 8 weeks, has been reported here. The absolute synthesis rate (ASR) is calculated as follows: FSR × pool size. The AT pool size can be most rigorously estimated from the adipocyte number in the tissue, which can be determined experimentally by the AT weight corrected for the mean adipocyte size. Alternatively, an estimate of the adipocyte size based on published values may be used when appropriate. It is important to note that when the AT pool size is in a steady state or near steady state, which is the usual state even in conditions like obesity (where the change in the total body AT mass over 8 weeks is typically quite small relative to the whole body AT mass), the FSR equals the fractional degradation rate (FDR) or death rate, which directly reveals the half-life of the cells: T1/2 = ln 2/FDR = 0.693/FDR9. Hence, assuming that the AT mass is in a relatively steady state during the 8 week 2H2O labeling period, the formation of new adipose cells is likely similar to the fraction of adipose cell deaths, which provides a measure of adipose cell turnover or replacement27. When there is a change in the pool size during the labeling period (non-steady state conditions), a non-steady state correction, such as the Steele equation29, can be used, which requires measuring or estimating the change in the pool size during the labeling period. Of note, the fractional synthesis "f" is still a valid measurement in a non-steady state, as the value of "f" represents the fraction of newly divided cells that are present. However, the conversion of "f" to a rate constant (FSR or FDR) would then require a non-steady state correction, such as the Steele equation. A strength of the 2H2O labeling protocol is that it can be applied to quantitatively measure adipose cell death, as losses in the total 2H enrichment in the adipose tissue pool after stopping the 2H2O consumption occur only by cell death9.
Although the need to isolate and purify the cells of interest could be viewed as a limitation of the protocol, some of the many advantages include the ease of 2H2O administration, the suitability of this approach for free-living conditions, the safety of the approach, and the fact that it provides an integrative assessment within the AT's natural milieu, which is not captured using in vitro approaches. Importantly, the 2H2O protocol is a practical approach that can be applied to assess in vivo kinetics in various other cell types, tissues, and disease states9.
A critical part of the experimental design is the dose and timing of the 2H2O administration9. The protocol presented employs an 8 week labeling period that involves the thrice-daily consumption of heavy water during week 1 (e.g., the priming period to approach the 2H2O enrichment plateau in the body water), followed by twice-daily consumption for the remaining weeks 2-8 to maintain relatively constant body water enrichment levels. Appropriate dosing and administration are important to provide the body 2H2O enrichment measurements, which are necessary to assess compliance with the protocol (protocol section 1) and to calculate the fraction of newly formed cells (protocol section 8). Of note, the 8 week labeling period has been shown to be sufficient and appropriate for measuring AT kinetics, despite the slow turnover of this tissue11,25. Hence, a benefit of this approach is that 2H2O is safe and suitable for consumption over a period of weeks or months, and the dose and timing of 2H2O administration can be modified based on the cell or tissue of interest, scientific question, and study objective to ensure proper 2H label incorporation9.
There are some points to consider in order to conduct follow-up studies. The half-life of 2H in body water is ~7-10 days in humans. After a subject discontinues 2H2O intake, the body water 2H reaches baseline after ~4-6 weeks (the dilution of 2H2O fits an exponential decay model)9. Although not a factor for cells with a rapid turnover (e.g., monocytes), the residual 2H labeling of adipocytes after the cessation of 2H2O intake is an important consideration when planning follow-up studies due to the slow turnover of these cells. Of note, the die-away of the label (e.g., adipocyte death) can be assessed and provide useful information, as the loss of the 2H label in the adipose cells occurs only if there is a loss of cells or cell death9. Before conducting follow-up studies, the enrichment of 2H in the adipose cells should be measured before the start of a 2H-labeling period to establish a "baseline" measurement when conducting follow-up studies.
The steps describing the DNA preparation, GC-MS analysis, and the calculation of newly formed cells (protocol sections 6-8) have been outlined and reported in extensive detail in Busch et al.9. The 2H enrichment in a (nearly) fully replaced reference cell type can be used in the equation to calculate the fraction of newly formed cells of interest (protocol section 8). This reference value represents the maximum 2H content possible in the DNA if the reference cell population comprises essentially all newly divided cells under the same 2H2O labeling conditions. Hence, in these analyses, the measurement in monocytes (nearly fully replaced cells) represents a reference cell marker of 2H2O exposure, and this was used to calculate the fraction of newly synthesized adipose cells. In the absence of a reference cell measure, body 2H2O enrichments can be used to estimate the 2H enrichment corresponding to 100% replaced cells (i.e., the theoretical maximum M1 enrichment; Figure 2). Of note, the use of monocyte, or other reference cell, 2H measures has an advantage over the body water measures, as the former measures account for the extent of incomplete 2H enrichment of the deoxyribonucleosides from 2H2O (due to incomplete 1H/2H exchange and/or kinetic isotope effects at the level of the metabolic precursors). The reference cell measures also serve to confirm the calculations using the theoretical maximum M1 enrichment.
This protocol describes the methods to measure in vivo AT kinetics and turnover rates in humans via the 2H2O labeling protocol. The data presented confirm this physiologic method as an innovative approach to assess in vivo AT kinetics and the association with obesity-related metabolic health outcomes27 in individuals with varying adipose distributions and body fat contents13, as well as in response to interventions, including diet, exercise, or pharmacological treatment26,28.
Disclosures
The authors have no conflicts of interest to declare.
Acknowledgements
The authors thank the Mass Spectrometry Core at Pennington Biomedical Research Center.
Materials
| Name | Company | Catalog Number | Comments |
| 1-methylimidazole | MilliporeSigma | 336092 | |
| 2H2O | Sigma Aldrich | ||
| Acetic anhydride | Aldridge | 539996 | |
| ACK Lysing Buffer (erythrocyte lysis buffer) | Quality Biological Inc (VWR) | 10128-802 | |
| Agilent 6890/5973 GC/MS | Agilent | ||
| Anti-human CD31 (PECAM-1) Biotin | Invitrogen | 13-0319-82 | |
| Anti-human CD34 Biotin | Invitrogen | 13-0349-82 | |
| Anti-human CD45 | BioLegend | 304004 | |
| Antibiotic Antimycotic Solution | MilliporeSigma | A5955 | |
| Collagenase type 1 | Worthington Biochemical Corporation | LS004196 | |
| Deoxyribose (2-deoxy d-ribose) | MilliporeSigma | 31170 | |
| Deuterium Oxide | MilliporeSigma | 756822 | |
| DB-225 column (30m, 0.25mm, 0.25um) | J&W Scientific | 122-2232 | |
| Dichloromethane (DCM) | MilliporeSigma | 34856 | |
| DNA standard (calf thymus DNA) | MilliporeSigma | D4764 | |
| Dneasy Blood and Tissue Kit (DNA extraction kit) | Qiagen | 69504 | |
| Easy Sep Human Biotin kit | Stem Cell Technologies | 17663 | |
| EasySep Human CD14 Positive Selection Cocktail | Stem Cell Technologies | 18058C | |
| Ethyl acetate | Fisher | EX0241-1 | |
| Falcon 5 mL Round Bottom Polystyrene Test Tube | VWR | 60819-295 | |
| Ficoll-Paque Plus | MilliporeSigma | GE17-1440-02 | |
| GC vials (2 mL) | Fisher | C-4011-1W | |
| GC vial inserts | Fisher | C-4011-631; C-4012-530 | |
| Glacial acetic acid | Fisher | AC14893-0010 | |
| Glass tubes (for hydrolysis) | Fisher | 14-959-35AA | |
| HEPES buffer | ThermoFisher | 15630080 | |
| Hyclone Water, molecular biology grade | Thomas Scientific | SH30538.02 | |
| MEM alpha | Fisher Scientific | 32561-037 | |
| PFBHA (o-(2, 3, 4, 5, 6)-penatfluorobenzylhydroxylamin hydrochloride) | MilliporeSigma | 194484 | |
| pH indicator strips | Fisher | 987618 | |
| Phosphatase acid | Calbiochem (VWR) | 80602-592 | |
| S1 nuclease (from Aspergillus oryzae) | MilliporeSigma | N5661 | |
| Sodium sulfate | MilliporeSigma | 23913 |
References
- Cypess, A. M. Reassessing human adipose tissue. The New England Journal of Medicine. 386 (8), 768-779 (2022).
- Cinti, S. The adipose organ at a glance. Disease Models & Mechanisms. 5 (5), 588-594 (2012).
- Sethi, J. K., Vidal-Puig, A. J. Thematic review series: Adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. Journal of Lipid Research. 48 (6), 1253-1262 (2007).
- White, U., Ravussin, E. Dynamics of adipose tissue turnover in human metabolic health and disease. Diabetologia. 62 (1), 17-23 (2019).
- Danforth, E. Failure of adipocyte differentiation causes type II diabetes mellitus. Nature Genetics. 26 (1), 13 (2000).
- Virtue, S., Vidal-Puig, A. Adipose tissue expandability, lipotoxicity and the metabolic syndrome--An allostatic perspective. Biochimica et Biophysica Acta. 1801 (3), 338-349 (2010).
- Arner, E., et al. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes. 59 (1), 105-109 (2010).
- Cinti, S., et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. Journal of Lipid Research. 46 (11), 2347-2355 (2005).
- Busch, R., Neese, R. A., Awada, M., Hayes, G. M., Hellerstein, M. K. Measurement of cell proliferation by heavy water labeling. Nature Protocols. 2 (12), 3045-3057 (2007).
- Neese, R. A., et al. Measurement in vivo of proliferation rates of slow turnover cells by 2H2O labeling of the deoxyribose moiety of DNA. Proceedings of the National Academy of Sciences of the United States of America. 99 (24), 15345-15350 (2002).
- Strawford, A., Antelo, F., Christiansen, M., Hellerstein, M. K. Adipose tissue triglyceride turnover, de novo lipogenesis, and cell proliferation in humans measured with 2H2O. American Journal of Physiology. Endocrinology and Metabolism. 286 (4), E577-E588 (2004).
- Macallan, D. C., et al. Measurement of cell proliferation by labeling of DNA with stable isotope-labeled glucose: Studies in vitro, in animals, and in humans. Proceedings of the National Academy of Sciences of the United States of America. 95 (2), 708-713 (1998).
- White, U. A., Fitch, M. D., Beyl, R. A., Hellerstein, M. K., Ravussin, E. Differences in in vivo cellular kinetics in abdominal and femoral subcutaneous adipose tissue in women. Diabetes. 65 (6), 1642-1647 (2016).
- Tchoukalova, Y. D., et al. In vivo adipogenesis in rats measured by cell kinetics in adipocytes and plastic-adherent stroma-vascular cells in response to high-fat diet and thiazolidinedione. Diabetes. 61 (1), 137-144 (2012).
- Jones, P. J., Leatherdale, S. T. Stable isotopes in clinical research: Safety reaffirmed. Clinical Science. 80 (4), 277-280 (1991).
- Bacchus-Souffan, C., et al. Relationship between CD4 T cell turnover, cellular differentiation and HIV persistence during ART. PLoS Pathogens. 17 (1), e1009214 (2021).
- Simonato, M., et al. Disaturated-phosphatidylcholine and surfactant protein-B turnover in human acute lung injury and in control patients. Respiratory Research. 12 (1), 36 (2011).
- Tremoy, G., et al. Measurements of water vapor isotope ratios with wavelength-scanned cavity ring-down spectroscopy technology: New insights and important caveats for deuterium excess measurements in tropical areas in comparison with isotope-ratio mass spectrometry. Rapid Communications in Mass Spectrometry. 25 (23), 3469-3480 (2011).
- dos Santos, T. H. R., Zucchi, M. D., Lemaire, T. J., de Azevedo, A. E. G., Viola, D. N. A statistical analysis of IRMS and CRDS methods in isotopic ratios of H-2/H-1 and O-18/O-16 in water. Sn Applied Sciences. 1, 664 (2019).
- Soleimani, M., Nadri, S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nature Protocols. 4 (1), 102-106 (2009).
- Hellerstein, M. K., Neese, R. A. Mass isotopomer distribution analysis at eight years: theoretical, analytic, and experimental considerations. The American Journal of Physiology. 276 (6), E1146-E1170 (1999).
- Patterson, B. W., Zhao, G., Klein, S. Improved accuracy and precision of gas chromatography/mass spectrometry measurements for metabolic tracers. Metabolism. 47 (6), 706-712 (1998).
- Mazzarello, A. N., Fitch, M., Hellerstein, M. K., Chiorazzi, N. Measurement of leukemic B-cell growth kinetics in patients with chronic lymphocytic leukemia. Methods in Molecular Biology. 1881, 129-151 (2019).
- Hellerstein, M. K., Neese, R. A. Mass isotopomer distribution analysis: A technique for measuring biosynthesis and turnover of polymers. The American Journal of Physiology. 263, E988-E1001 (1992).
- Spalding, K. L., et al. Dynamics of fat cell turnover in humans. Nature. 453 (7196), 783-787 (2008).
- Allerton, T. D., et al. Exercise reduced the formation of new adipocytes in the adipose tissue of mice in vivo. PLoS One. 16 (1), e0244804 (2021).
- White, U. A., Fitch, M. D., Beyl, R. A., Hellerstein, M. K., Ravussin, E. Association of in vivo adipose tissue cellular kinetics with markers of metabolic health in humans. The Journal of Clinical Endocrinology and Metabolism. 102 (7), 2171-2178 (2017).
- White, U., Fitch, M. D., Beyl, R. A., Hellerstein, M. K., Ravussin, E. Adipose depot-specific effects of 16 weeks of pioglitazone on in vivo adipogenesis in women with obesity: A randomised controlled trial. Diabetologia. 64 (1), 159-167 (2021).
- Steele, R. Influences of glucose loading and of injected insulin on hepatic glucose output. Annals of the New York Academy of Sciences. 82, 420-430 (1959).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved