Method Article
A Medical Decision Support Platform for Identifying Thrombospondin 1 in Non-alcoholic Fatty Liver Disease via Immuno-Infiltration Analysis
In This Article
Summary
This study investigated the relationships between non-alcoholic fatty liver disease (NAFLD) and myocardial infarction (MI) through co-expressed genes, identifying Thrombospondin 1 (THBS1) as a biomarker. Immuno-infiltration analysis revealed CD8+ T cells and neutrophils as key factors, with THBS1 showing potential as a diagnostic tool for NAFLD and MI.
Abstract
Non-alcoholic fatty liver disease (NAFLD) and myocardial infarction (MI) are two major health burdens with significant prevalence and mortality. This study aimed to explore the co-expressed genes to understand the relationship between NAFLD and MI and identify potential crucial biomarkers of NAFLD-related MI using bioinformatics and machine learning. Functional enrichment analysis was conducted, a co-protein-protein interaction (PPI) network diagram was constructed, and support vector machine-recursive feature elimination (SVM-RFE) and least absolute shrinkage and selection operator (LASSO) techniques were employed to identify one differentially expressed gene (DEG), Thrombospondin 1 (THBS1). THBS1 demonstrated strong performance in distinguishing NAFLD patients (AUC = 0.981) and MI patients (AUC = 0.900). Immuno-infiltration analysis revealed significantly lower CD8+ T cells and higher neutrophil levels in patients with NAFLD and MI. CD8+ T cells and neutrophils were effective in distinguishing NAFLD/MI from healthy controls. Correlation analysis showed that THBS1 was positively correlated with CCR (chemokine receptor), MHC class (major histocompatibility complex class), neutrophils, parainflammation, and Tfh (follicular helper T cells), and negatively correlated with CD8+ T cells, cytolytic activity, and TIL (tumor-infiltrating lymphocytes) in NAFLD and MI patients. THBS1 emerged as a novel biomarker for diagnosing NAFLD/MI in comparison to healthy controls. The results indicate that CD8+ T cells and neutrophils could serve as inflammatory immune features for differentiating patients with NAFLD/MI from healthy individuals.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a major public health issue with a prevalence of 25%-30%1. It has been reported that the prevalence of NAFLD is high among patients with diabetes2. However, the significance of NAFLD in non-diabetic patients is not yet clear. Studies have suggested that NAFLD plays an independent role in the pathogenesis of atherosclerosis3,4. Additionally, a meta-analysis has shown that NAFLD is closely associated with coronary artery calcification, endothelial dysfunction, and atherosclerosis, and has emerged as an independent cardiovascular risk factor5. The connection between NAFLD and cardiovascular disease still requires further investigation.
Myocardial infarction (MI) is a catastrophic disease that threatens health and imposes a significant economic burden on patients and their families worldwide6. MI is also a major cause of death in patients with NAFLD. A clinical study published in the British Medical Journal has shown that the risk of myocardial infarction in NAFLD patients is 1.17 times greater than in non-NAFLD patients7,8. Some studies have identified molecular pathways, including inflammation, oxidative stress, and lipid metabolism, that contribute to NAFLD-related MI9,10,11. However, the underlying mechanisms linking NAFLD and MI remain unclear. It is crucial to identify novel biomarkers related to the prognosis of NAFLD and MI.
The increasing prevalence of NAFLD, which affects a broad segment of the population, underscores a significant public health issue, particularly given its association with diabetes. However, the impact of NAFLD on non-diabetic patients remains poorly understood. NAFLD has been implicated in the pathogenesis of atherosclerosis and is recognized as an independent cardiovascular risk factor, being closely linked to coronary artery calcification, endothelial dysfunction, and atherosclerosis. Despite these associations, the precise mechanisms bridging NAFLD and cardiovascular diseases such as myocardial infarction (MI) require further elucidation. MI is one of the leading causes of mortality worldwide and imposes a significant economic burden. The risk of MI in NAFLD patients is notably higher than in those without NAFLD, highlighting the need for a deeper understanding of the molecular pathways connecting these conditions. While inflammation, oxidative stress, and lipid metabolism have been suggested as contributing factors, the exact mechanisms remain unclear. There is an urgent need to identify novel biomarkers that can provide insights into the prognosis and management of NAFLD-related MI.
Consequently, in this study, RNA microarray datasets for NAFLD and MI were downloaded from the National Center for Biotechnology Information-Gene Expression Omnibus (NCBI-GEO, see Table of Materials) to identify and analyze the interaction of differentially expressed genes (DEGs) between NAFLD and MI. Enrichment analysis, protein-protein interaction (PPI) network diagram construction, support vector machine-recursive feature elimination (SVM-RFE), and least absolute shrinkage and selection operator (LASSO) algorithms were used to identify hub genes12,13,14,15,16,17,18,19. Immuno-infiltration analysis was performed to examine immune cells in NAFLD and MI patients. Ultimately, these methods were integrated to elucidate the relationship between NAFLD and MI. Figure 1 illustrates the design sequence followed in this study. By combining bioinformatics, machine learning, and immuno-infiltration analysis, this study aims to contribute to the development of a novel medical decision support platform.
The key contributions of this article are: (1) Identification of co-expressed genes: The study highlights the relationship between NAFLD and MI by identifying co-expressed genes, offering a deeper understanding of the molecular links between these two conditions. (2) Application of bioinformatics and machine learning: Using bioinformatics and machine learning techniques, including support vector machine-recursive feature elimination (SVM-RFE)17 and least absolute shrinkage and selection operator (LASSO)19, the study identifies THBS1 as a differentially expressed gene. THBS1 demonstrates high performance in distinguishing NAFLD and MI patients from healthy controls. (3) Immuno-infiltration analysis: The study conducts an immuno-infiltration analysis, revealing significantly lower levels of CD8+ T cells and higher levels of neutrophils in patients with NAFLD and MI. (4) Correlation analysis: The research demonstrates that THBS1 is positively correlated with several immune factors, including CCR (chemokine receptor), MHC class I (major histocompatibility complex class I), neutrophils, parainflammation, and Tfh (follicular helper T) cells. It is negatively correlated with CD8+ T cells, cytolytic activity, and tumor-infiltrating lymphocytes (TIL).
Protocol
The details of the databases, weblinks, and software/packages used are listed in the Table of Materials. The simulation parameters used are provided in Table 1.
1. Obtaining RNA microarray datasets
- Download the myocardial infarction (MI) dataset, GSE66360, from the NCBI-GEO database.
- Download the non-alcoholic fatty liver disease (NAFLD) dataset, GSE89632, from the NCBI-GEO database.
- Ensure that the downloaded datasets include 49 MI samples, 50 healthy controls for GSE66360, 39 NAFLD samples, and 24 healthy controls for GSE89632.
2. Identification of DEGs
- Data preprocessing and DEG identification
- Load the datasets into RStudio using appropriate R functions.
- Utilize the R package limma to identify DEGs12 with the following thresholds: P < 0.05P and | log FC | > 1.5.
- Use the R packages pheatmap, ggplot2, and Venn to generate heatmaps and Venn diagrams for visualizing DEGs.
- Create visualizations
- Use pheatmap to generate heatmaps of DEGs.
- Use ggplot2 to create Venn diagrams showing the overlap of DEGs between NAFLD and MI.
3. Enrichment analysis
- Perform GO, KEGG, and DO enrichment analysis
- Load DEGs into the R package clusterProfiler13.
- Conduct Gene Ontology (GO) enrichment analysis to categorize DEGs into molecular functions, biological processes, and cellular components.
- Perform Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis to map DEGs onto biochemical pathways.
- Use Disease Ontology (DO) enrichment analysis to link DEGs to specific clinical states.
4. PPI analysis by constructing PPI networks
- Use the STRING online platform14 to build protein-protein interaction (PPI) network diagrams of co-DEGs with a confidence score of 0.4.
- Visualize the PPI networks using Cytoscape15.
5. Candidate hub DEGs screening by applying machine learning algorithms
- Use SVM-RFE (Support Vector Machine-Recursive Feature Elimination) and LASSO (Least Absolute Shrinkage and Selection Operator) regression algorithms in RStudio to select the most relevant DEGs16,17,18.
- Ensure that SVM-RFE ranks and removes features iteratively, while LASSO regression19 applies regularization to identify a sparse set of DEGs.
6. ROC curve construction for assessing diagnostic performance
- Use RStudio to conduct ROC (Receiver Operating Characteristic) curve analysis20.
- Calculate the area under the curve (AUC) for crucial co-DEGs.
7. Immuno-infiltration analysis to explore immune infiltration
- Perform single-sample Gene Set Enrichment Analysis (ssGSEA) using the R packages GSVA and GSEABase to analyze immune infiltration in NAFLD and MI21.
- Compare the relative abundance of immune cell types between NAFLD and MI samples and healthy controls.
8. Statistical analysis
- Perform all statistical analyses in RStudio.
- Apply Pearson correlation analysis to examine gene co-expression patterns.
- Ensure statistical significance with P < 0.05.
NOTE: Ensure that all software and R packages are properly installed and updated to their latest versions before starting the protocol.
Results
The key findings of the proposed study are presented here, encompassing various analyses conducted to elucidate the molecular mechanisms underlying NAFLD and MI.
Identification of DEGs
In the GSE89632 dataset, 76 up-regulated and 20 down-regulated genes were identified as NAFLD-DEGs (Figure 2B,D), while the GSE66360 dataset revealed 118 up-regulated and 8 down-regulated genes as MI-DEGs (Figure 2C,E). Subsequently, co-DEGs were derived from the overlap between NAFLD-DEGs and MI-DEGs, identifying 13 up-regulated DEGs and 1 down-regulated DEG among the co-DEGs (Figure 2A).
Identifying DEGs unique to NAFLD and MI, as well as those common to both disorders, provides valuable insights into the molecular processes underlying these illnesses. Understanding the distinct and shared gene expression patterns linked to both conditions helps clarify the pathophysiology of NAFLD and MI and may identify potential molecular targets for therapeutic intervention. Further investigation into the co-DEGs could reveal critical regulators or pathways essential to the interaction between these two disorders, facilitating the development of targeted therapies aimed at mitigating the negative health outcomes associated with NAFLD and MI.
Enrichment analysis
GO analysis revealed that NAFLD DEGs were primarily enriched in the following biological processes (BP): fat cell differentiation, response to lipopolysaccharide, and multicellular organism processes (p < 0.05, Figure 3A). For cellular components (CC), NAFLD DEGs were mainly associated with the phosphatidylinositol 3-kinase complex, secretory granule lumen, and cytoplasmic vesicle lumen (p < 0.05, Figure 3A). In terms of molecular function (MF), the key activities included phosphatidylinositol-3-kinase regulator activity, DNA-binding transcription activator activity (RNA polymerase-specific), and general DNA-binding transcription activator activity (p < 0.05, Figure 3A).
For MI-DEGs, the BP analysis showed enrichment in leukocyte chemotaxis, myeloid leukocyte activation, and cell chemotaxis (p < 0.05, Figure 3B). The CC analysis highlighted enrichment in tertiary granules, ficolin-1-rich granules, and secretory granule lumen (p < 0.05, Figure 3B). MF analysis revealed enrichment in pattern recognition receptor activity, RAGE receptor binding, and immune receptor activity (p < 0.05, Figure 3B).
KEGG enrichment analysis indicated that NAFLD-DEGs were significantly enriched in the following pathways: IL-17, AGE-RAGE, TNF, osteoclast differentiation, malaria, rheumatoid arthritis, Chagas disease, and JAK-STAT signaling pathways (p < 0.05, Figure 3C). MI-DEGs were enriched in IL-17, TNF, lipid metabolism and atherosclerosis, toll-like receptor signaling, C-type lectin receptor signaling, legionellosis, rheumatoid arthritis, and NF-κB signaling pathways (p < 0.05, Figure 3D). Disease Ontology (DO) enrichment analysis demonstrated that the top three DEGs for both NAFLD and MI were significantly associated with atherosclerosis, arteriosclerotic cardiovascular disease, and arteriosclerosis (p < 0.05, Figure 3E-F).
PPI network diagrams analysis
In the PPI network diagram for NAFLD, 92 nodes and 250 edges were identified, reflecting a complex network of interactions among proteins associated with this condition. Similarly, the PPI network diagram for MI revealed 117 nodes and 587 edges, indicating a more intricate network characteristic of the molecular landscape of myocardial infarction. Additionally, the PPI network diagrams for co-DEGs identified 14 nodes and 21 edges, highlighting potential key interactions among proteins shared between NAFLD and MI (Figure 4A-F).
PPI networks are valuable for constructing and analyzing therapeutic targets and provide important insights into the molecular connections underlying NAFLD and MI. The unique network topologies observed in NAFLD and MI emphasize the distinct pathophysiological mechanisms involved in these diseases. Moreover, the discovery of shared connections between co-DEGs suggests potential shared pathways or regulatory networks that mediate the interaction between MI and NAFLD. Further research into these connections could reveal novel molecular targets for intervention and therapy development, potentially offering new treatment options for these complex illnesses.
Candidate hub DEGs screening
The SVM-RFE algorithm17 was employed to screen for potential crucial DEGs, resulting in the identification of 6 genes in GSE89632 and 4 genes in GSE66360 (Figure 5A,B). Similarly, the LASSO algorithm19 was used to identify candidate crucial DEGs, yielding 4 genes in GSE89632 and 5 genes in GSE66360 (Figure 5C,D). Notably, a Venn diagram depicted the intersection of the hub DEG (THBS1) identified by SVM-RFE, LASSO, and co-PPI network analyses, underscoring its significance in both NAFLD and MI pathogenesis (Figure 5F).
These advanced machine learning algorithms provided a robust framework for identifying key DEGs associated with MI and NAFLD and offered valuable insights into their potential as therapeutic or diagnostic targets. The identification of THBS1 as a hub gene through multiple analytical techniques highlights its importance in the molecular pathways underlying both diseases. Further research into the functional significance of THBS1 and its interactions within the co-PPI network could elucidate novel mechanisms linking NAFLD and MI, potentially paving the way for targeted therapies and precision medicine strategies.
Diagnostic value of one hub DEGs
ROC analysis was conducted to assess the diagnostic efficiency of the identified hub DEGs in both NAFLD and MI datasets. As depicted in Figure 6A,B, THBS1 demonstrated high discriminatory power, with an AUC of 0.981 (95% CI: 0.949-1.000) in NAFLD patients and 0.900 (95% CI: 0.834-0.956) in MI patients. The strong performance of THBS1 as a diagnostic biomarker highlights its potential usefulness in differentiating between NAFLD, MI, and healthy controls.
These results suggest that THBS1 could serve as a valuable marker for disease state, offering clinicians a useful tool for risk assessment and early identification. To facilitate the integration of THBS1 into clinical practice and improve patient care and management, further validation studies are needed to confirm its diagnostic accuracy across diverse patient populations and clinical settings.
Immune infiltration analysis
ssGSEA was conducted to analyze 29 immune-related genes between NAFLD/MI and the control group (CON). Compared to the CON group, immune cell infiltration was significantly increased in the NAFLD and MI groups, particularly for CCR, MHC class I, neutrophils, parainflammation, and Tfh cells (p < 0.05, Figure 7A,B). Conversely, immune cell infiltration decreased notably in the NAFLD and MI groups relative to the CON group, especially for CD8+ T cells, cytolytic activity, and tumor-infiltrating lymphocytes (TIL) (p < 0.05, Figure 7C,D).
These results suggest dysregulation of immune cell infiltration in both NAFLD and MI, highlighting the complex immunological landscape associated with these conditions. The observed changes in immune cell composition provide valuable insights into the immunological processes underlying the development and pathophysiology of these diseases. Further understanding of these immune characteristics could reveal new therapeutic targets for intervention and support the development of individualized treatment plans based on the immunological profiles of patients with NAFLD and MI.
Figure 1: The design sequence of the medical decision support system for identifying THBS1. Please click here to view a larger version of this figure.

Figure 2: Venn diagrams, volcano diagrams, and heat maps. (A) Venn diagram for GSE89632 and GSE66360. (B) A volcano diagram of differentially expressed genes (DEGs) in GSE89632. (C) A volcano diagram of DEGs in GSE66360. (D) A heat map diagram of DEGs in GSE89632. (E) A heat map diagram of DEGs in GSE66360. Please click here to view a larger version of this figure.

Figure 3: Enrichment analysis of differentially expressed genes (DEGs). (A) The top five NALFD- DEGs enriched gene ontology (GO) in biological process (BP), cellular component (CC), and molecular function (MF). (B) The top five MI-DEGs enriched GO in BP, CC and MF. (C) The top eight NALFD-DEGs enriched Kyoto encyclopedia of genes and genomes (KEGG) signaling pathways. (D) The top eight MI-DEGs enriched KEGG signaling pathways. (E) The top five NALFD-DEGs enriched disease ontology (DO). (F) The top five MI-DEGs enriched DO. Please click here to view a larger version of this figure.
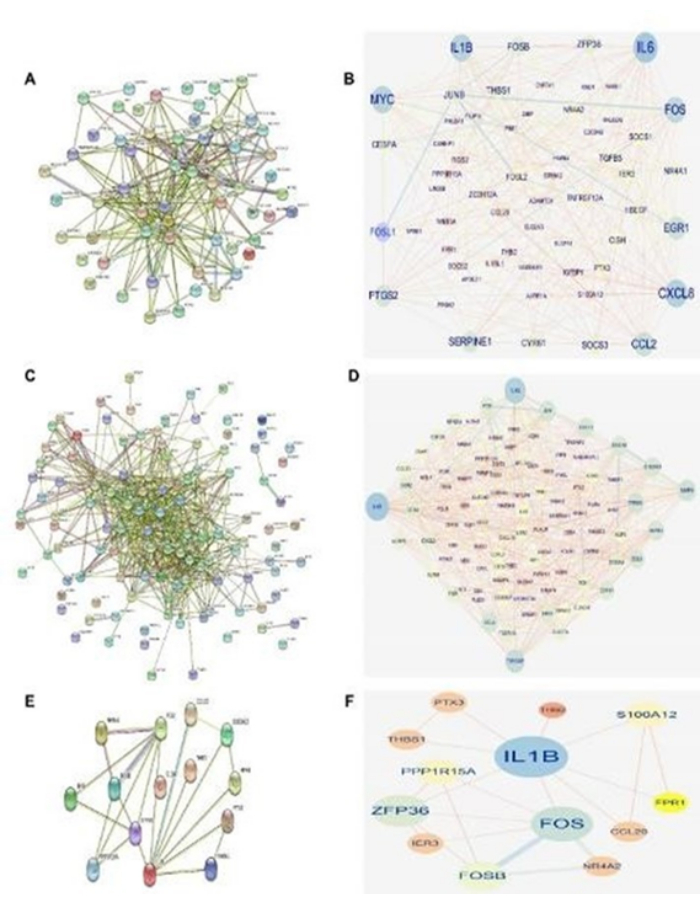
Figure 4: Protein-protein interaction (PPI) network diagrams. (A) A PPI network diagram of non-alcoholic fatty liver disease (NAFLD) obtained from the String online platform. (B) A PPI network diagram of NAFLD obtained from Cytoscape. (C) A PPI network diagram of myocardial infarction (MI) obtained from String online platform. (D) A PPI network diagram of MI obtained Cytoscape. (E) A PPI network diagram of protein interactions. (F) A PPI network diagram of IL1B and FOS. Please click here to view a larger version of this figure.

Figure 5: Candidate hub DEGs screening. (A) Six crucial DEGs obtained by SVM-RFE in NAFLD. (B) Four crucial DEGs obtained by SVM-RFE in myocardial infarction (MI). (C) Four crucial DEGs obtained by LASSO in NAFLD. (D) Five crucial DEGs obtained by LASSO in MI. (E) Only one DEG acquired from the above results in the Venn diagram. Please click here to view a larger version of this figure.

Figure 6: Diagnostic value of one hub differentially expressed genes (DEGs). (A) ROC curves in NAFLD. (B) ROC curves in MI. Please click here to view a larger version of this figure.

Figure 7: Distribution of infiltrating immune cells and correlation between THBS1 and infiltrating immune cells in non-alcoholic fatty liver disease (NAFLD) and myocardial infarction (MI). (A) Distribution of infiltrating immune cells in NAFLD. (B) Distribution of infiltrating immune cells in MI. (C) Correlation between THBS1 and infiltrating immune cells in NAFLD. (D) Correlation between THBS1 and infiltrating immune cells in MI. Please click here to view a larger version of this figure.
| Abbreviation | Nomenclature |
| AUC | Area Under Curve |
| BP | Biological Process |
| CC | Cellular Component |
| CON | Controls |
| DEG | Differentially Expressed Genes |
| DO | Disease Ontology |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LASSO | Least Absolute Shrinkage and Selection Operator |
| MF | Molecular Function |
| MI | Myocardial Infarction |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| PPI | Protein-Protein Interaction |
| ROC | Receiver Operator Characteristic Curves |
| ssGSEA | single-sample Gene Set Enrichment Analysis |
| SVM-RFE | Support Vector Machine-Recursive Feature Elimination |
| THBS1 | Thrombospondin 1 |
Table 1: Simulation parameters used in the experimentation.
Discussion
The method described in this study has significant implications for research into the molecular mechanisms underlying NAFLD and MI. By identifying key biomarkers such as THBS1, the proposed protocol offers potential targets for both diagnostic and therapeutic interventions. This approach can be extended to other complex diseases involving multiple pathways and immune responses, facilitating the discovery of novel biomarkers and therapeutic targets. Moreover, the integration of bioinformatics and machine learning techniques provides a versatile framework for personalized medicine, allowing for tailored treatment strategies based on individual molecular profiles. This method can also be adapted for use in clinical settings to enhance diagnostic accuracy and improve patient outcomes through targeted interventions.
NAFLD and myocardial infarction (MI) are two major health burdens with significant prevalence and mortality. NAFLD is characterized by the accumulation of lipids in the liver1. Increasing evidence suggests that NAFLD adversely affects not only the structural integrity of the heart but also its function, particularly in the context of MI22,23,24. Furthermore, there is compelling evidence indicating a strong correlation between NAFLD and atherosclerosis. Studies have found that the prevalence and severity of NAFLD in patients with non-diabetic MI are linked to coronary artery disease25. Moderate to severe NAFLD has been shown to be an independent risk factor, increasing the risk of multiple coronary artery lesions threefold4. However, these studies primarily focus on NAFLD and MI, leaving the underlying mechanisms of the association between NAFLD and atherosclerosis still unclear. Thus, it is crucial to explore these mechanisms to prevent NAFLD-related MI.
In this study, two datasets were obtained from the NCBI-GEO. The analysis revealed 13 up-regulated DEGs and 1 down-regulated DEG between NAFLD and MI. Functional enrichment analysis was performed, a co-PPI network diagram was constructed, and two machine-learning algorithms were employed to identify hub DEGs. As a result, THBS1 was identified as a novel biomarker with outstanding diagnostic efficiency for both NAFLD and MI. Support Vector Machine (SVM) is a supervised machine learning technique widely used for classification and regression17. Recursive feature elimination (RFE) helps select the most significant genes from the sample. The LASSO regression algorithm19 applies regularization to improve prediction accuracy. By combining these algorithms with co-DEGs, we were able to screen for the optimal DEGs.
Based on the analysis using two machine learning algorithms and co-DEGs, Thrombospondin 1 (THBS1 or TSP1) was identified as a key diagnostic hub DEG. THBS1 is a member of the extracellular matrix proteins and was first detected in platelets in response to thrombin stimulation26. Various cells, including endothelial cells, hepatic stellate cells, and smooth muscle cells, can produce and secrete THBS1 into the extracellular space27,28,29. THBS1 interacts with CD36, a known ligand30, and exerts an anti-angiogenic effect by regulating CD3629,31, underscoring its significant role in cardiovascular disease. Additionally, THBS1 can regulate angiogenesis and platelet aggregation through its interactions with receptors and proteins such as CD47, transforming growth factor-β, and integrins32. This suggests that THBS1 may play a crucial role in thrombus formation, vulnerable plaque rupture, and subsequent ventricular remodeling during MI33,34,35.
Furthermore, NAFLD development is closely associated with the synthesis of secreted proteins in circulation. A pivotal study published in EBioMedicine demonstrated that THBS1, as a secreted protein, could serve as a novel biomarker for NAFLD. This study also found that exogenous THBS1 administration improves hepatic lipid deposition by inhibiting lipid synthesis pathways36. Notably, CD36 was identified as a key receptor for THBS1's effects against NAFLD. Additionally, ABT-526, a peptide analog of THBS1, has been shown to inhibit lipid synthesis in hepatocytes. These findings provide new insights into the diagnosis and treatment of NAFLD and suggest that THBS1 could be a novel biomarker for both NAFLD and MI, offering a fast, simple, and accessible diagnostic tool.
Recent evidence highlights the critical role of immune-inflammatory responses in the progression of NAFLD and MI. Key features include cytokine expression, immune regulation, and activation of the neuroendocrine system. Wabitsch et al. demonstrated that metformin confers hepatoprotection by inhibiting the expression of CD8+ T cells in NAFLD mice37. Dolejsi et al. reported a decrease in CD8+ T cells and a significant reduction in myocardial repair capacity38. Qin et al. found that neutrophils, as part of the systemic immune-inflammation index, are associated with urinary albumin excretion in NAFLD patients39. Zhang et al. indicated that neutrophil infiltration and degranulation are involved in both injury and recovery processes during MI27.
In alignment with these findings, the current results revealed increased infiltration of CCR, MHC class I, neutrophils, para-inflammation, and Tfh cells, while CD8+ T cells, cytolytic activity, and TIL were decreased in NAFLD and MI patients compared to healthy controls. Moreover, correlation analysis showed that THBS1 was positively correlated with CCR, MHC class I, neutrophils, parainflammation, and Tfh cells, and negatively correlated with CD8+ T cells, cytolytic activity, and TIL in NAFLD and MI patients. These observations suggest that THBS1 may contribute to the modulation of immune responses by increasing CCR, MHC class I, neutrophils, parainflammation, and Tfh cells, while decreasing CD8+ T cells, cytolytic activity, and TIL during the development and progression of NAFLD and MI. However, these speculations warrant further investigation to confirm the precise role of THBS1 in these processes.
This study aimed to elucidate the molecular connections between NAFLD and MI using comprehensive bioinformatics and machine learning approaches. The findings identified 96 differentially expressed genes (DEGs) in NAFLD and 126 in MI, with 14 DEGs overlapping between the two conditions. Functional enrichment analysis revealed that these DEGs are involved in critical biological processes and pathways, such as fat cell differentiation, leukocyte chemotaxis, and various signaling pathways, including IL-17, TNF, and NF-κB. These results highlight the intricate interplay of inflammatory and metabolic pathways in NAFLD and MI. The construction of protein-protein interaction (PPI) network diagrams, combined with machine learning analyses (SVM-RFE and LASSO), identified THBS1 as a key hub gene. THBS1 demonstrated excellent diagnostic performance, with an area under the curve (AUC) of 0.981 for NAFLD and 0.900 for MI, suggesting its potential as a robust biomarker for both conditions.
Furthermore, immuno-infiltration analysis revealed distinct immune cell profiles in NAFLD and MI patients compared to healthy controls. Specifically, NAFLD and MI patients exhibited increased infiltration of CCR, MHC class I, neutrophils, parainflammation, and Tfh cells, while showing decreased levels of CD8+ T cells, cytolytic activity, and TIL. These immune features underscore the inflammatory nature of both conditions and highlight the role of THBS1 in modulating immune responses.
This study provides new insights into the shared molecular mechanisms underlying NAFLD and MI, with THBS1 emerging as a novel biomarker for diagnosis and a potential therapeutic target. The findings pave the way for future research into targeted interventions and personalized treatment strategies for patients with these interconnected conditions. By integrating bioinformatics, machine learning, and immuno-infiltration analysis, the research contributes to the development of a medical decision support platform aimed at improving the clinical management of NAFLD and MI.
Disclosures
None.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 62271511, U21A200949), Yucai Foundation of General Hospital of the Southern Theatre Command (2022NZC011), the Guangzhou Science and Technology Program Project (2023A03J0170), the National Clinical Research Center for Geriatrics (NCRCG-PLAGH-2023006) and Guangdong Basic and Applied Basic Research Foundation (No.2020A1515010288, No.2021A1515220101).
Materials
| Name | Company | Catalog Number | Comments |
| Cytoscape | Cytoscape Consortium | Version 3.6.1 | Used for visualizing protein-protein interaction (PPI) networks |
| MI dataset GSE66360 (https://www.ncbi.nlm.nih.gov/geo/). | NCBI-GEO database | - | To collect RNA microarray datasets for analysis |
| R package clusterProfiler | Bioconductor | - | Used for GO, KEGG, and DO enrichment analyses |
| R package ggplot2 | CRAN | - | Used for creating Venn diagrams and other visualizations |
| R package GSEABase | Bioconductor | - | Used in conjunction with GSVA for gene set enrichment analysis |
| R package GSVA | Bioconductor | - | Used for single-sample gene set enrichment analysis (ssGSEA) |
| R package limma | Bioconductor | - | Used for identifying differentially expressed genes (DEGs) |
| R package pheatmap | CRAN | - | Used for generating heatmaps |
| R package venn | CRAN | - | Used for creating Venn diagrams |
| RNA microarray datasets (GSE66360, GSE89632) | NCBI-GEO | - | Publicly available RNA microarray datasets used for analysis |
| RStudio | RStudio, PBC | Version 1.4.1717 | Integrated development environment for R |
| String database | STRING (www.string-db.org/) | - | Online tool for constructing PPI networks |
References
- de Alwis, N. M. W., Day, C. P. Non-alcoholic fatty liver disease: The mist gradually clears. J Hepatol. 48 (Suppl), S104-S112 (2008).
- Adams, L. A., Anstee, Q. M., Tilg, H., Targher, G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 66 (6), 1138-1153 (2017).
- Bhatia, L. S., Curzen, N. P., Calder, P. C., Byrne, C. D. Non-alcoholic fatty liver disease: A new and important cardiovascular risk factor. Eur Heart J. 33 (9), 1190-1200 (2012).
- Boddi, M., et al. Non-alcoholic fatty liver in non-diabetic patients with acute coronary syndromes. Eur J Clin Invest. 43 (4), 429-438 (2013).
- Oni, E. T., et al. A systematic review: Burden and severity of subclinical cardiovascular disease among those with non-alcoholic fatty liver; should we care. Atherosclerosis. 230 (2), 258-267 (2013).
- Reed, G. W., Rossi, J. E., Cannon, C. P. Acute myocardial infarction. Lancet. 389 (10070), 197-210 (2017).
- Stahl, E. P., et al. Non-alcoholic fatty liver disease and the heart: JACC state-of-the-art review. J Am Coll Cardiol. 73 (8), 948-963 (2019).
- Alexander, M., et al. Non-alcoholic fatty liver disease and risk of incident acute myocardial infarction and stroke: findings from matched cohort study of 18 million European adults. BMJ. 367, 1934578X221093907 (2019).
- Cobbina, E., Akhlaghi, F. Non-alcoholic fatty liver disease (NAFLD)-pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metab Rev. 49 (2), 197-211 (2017).
- Pawlak, M., Lefebvre, P., Staels, B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 62 (3), 720-733 (2015).
- Katsiki, N., Mikhailidis, D. P., Mantzoros, C. S. Non-alcoholic fatty liver disease and dyslipidemia: An update. Metabolism. 65 (8), 1109-1123 (2016).
- Ritchie, M. E., et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43 (7), e47 (2015).
- Yu, G., Wang, L. G., Han, Y., He, Q. Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics. 16 (5), 284-287 (2012).
- Szklarczyk, D., et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 49 (D1), D605-D612 (2021).
- Sriroopreddy, R., Sajeed, R., Raghuraman, P., Sudandiradoss, C. Differentially expressed gene (DEG) based protein-protein interaction (PPI) network identifies a spectrum of gene interactome, transcriptome and correlated miRNA in nondisjunction Down syndrome. Int J Biol Macromol. 122, 1080-1089 (2019).
- Engebretsen, S., Bohlin, J. Statistical predictions with glmnet. Clin Epigenetics. 11, 123 (2019).
- Ma, H., Ding, F., Wang, Y. A novel multi-innovation gradient support vector machine regression method. ISA Trans. 130, 343-359 (2022).
- Han, Y., Huang, L., Zhou, F. A dynamic recursive feature elimination framework (dRFE) to further refine a set of OMIC biomarkers. Bioinformatics. 37 (12), 2183-2189 (2021).
- Colombani, C., et al. Application of Bayesian least absolute shrinkage and selection operator (LASSO) and BayesCπ methods for genomic selection in French Holstein and Montbéliarde breeds. J Dairy Sci. 96 (2), 575-591 (2013).
- Gamper, M., et al. Gene expression profile of bladder tissue of patients with ulcerative interstitial cystitis. BMC Genomics. 10 (1), 1-17 (2009).
- Hänzelmann, S., Castelo, R., Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics. 14, 1-15 (2013).
- Valbusa, F., et al. Non-alcoholic fatty liver disease and increased risk of all-cause mortality in elderly patients admitted for acute heart failure. Int J Cardiol. 265, 162-168 (2018).
- Zhang, Q., et al. Identification of hub biomarkers of myocardial infarction by single-cell sequencing, bioinformatics, and machine learning. Front Cardiovasc Med. 9, 939972 (2022).
- Zhang, Q., et al. 7-Hydroxyflavone alleviates myocardial ischemia/reperfusion injury in rats by regulating inflammation. Molecules. 27 (17), 5371 (2022).
- Zhang, Q., Guo, Y., Zhang, D. Network pharmacology integrated with molecular docking elucidates the mechanism of wuwei yuganzi san for the treatment of coronary heart disease. Nat Prod Commun. 17 (1), 1934578X221093907 (2022).
- Baenziger, N. L., Brodie, G., Majerus, P. W. A thrombin-sensitive protein of human platelet membranes. Proc Natl Acad Sci USA. 68 (2), 240-243 (1971).
- Zhang, N., Aiyasiding, X., Li, W. J., Liao, H. H., Tang, Q. Z. Neutrophil degranulation and myocardial infarction. Cell Commun Signal. 20 (1), 50 (2022).
- Lawler, J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J Cell Mol Med. 6 (1), 1-12 (2002).
- Kaur, S., et al. Functions of thrombospondin-1 in the tumor microenvironment. Int J Mol Sci. 22 (9), 4570 (2021).
- Asch, A. S., et al. Isolation of the thrombospondin membrane receptor. J Clin Invest. 79 (4), 1054-1061 (1987).
- Armstrong, L. C., Bornstein, P. Thrombospondins 1 and 2 function as inhibitors of angiogenesis. Matrix Biol. 22 (1), 63-71 (2003).
- Roberts, D. D., Isenberg, J. S. CD47 and thrombospondin-1 regulation of mitochondria, metabolism, and diabetes. Am J Physiol Cell Physiol. 321 (1), C201-C213 (2021).
- Emre, A., et al. Impact of non-alcoholic fatty liver disease on myocardial perfusion in non-diabetic patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Am J Cardiol. 116 (11), 1810-1814 (2015).
- Mouton, A. J., et al. Fibroblast polarization over the myocardial infarction time continuum shifts roles from inflammation to angiogenesis. Basic Res Cardiol. 114 (1), 1-16 (2019).
- Liu, Y., et al. Atherosclerotic conditions promote the packaging of functional microRNA-92a-3p into endothelial microvesicles. Circ Res. 124 (4), 575-587 (2019).
- Bai, J., et al. Thrombospondin 1 improves hepatic steatosis in diet-induced insulin-resistant mice and is associated with hepatic fat content in humans. EBioMedicine. 57 (1), 102-111 (2020).
- Wabitsch, S., et al. Metformin treatment rescues CD8+ T-cell response to immune checkpoint inhibitor therapy in mice with NAFLD. J Hepatol. 77 (4), 748-760 (2022).
- Dolejsi, T., et al. Adult T-cells impair neonatal cardiac regeneration. Eur Heart J. 43 (27), 2698-2709 (2022).
- Qin, Z., et al. Systemic immune-inflammation index is associated with increased urinary albumin excretion: a population-based study. Front Immunol. 13, 863640 (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved