Method Article
Bilateral Renal Ischemia-Reperfusion Model for Acute Kidney Injury in Mice
In This Article
Summary
The ischemia-reperfusion injury (IRI) model can be used at different stages of acute kidney injury (AKI) development, especially during AKI to chronic kidney disease (CKD) progression. Here, we describe the procedure for IRI model development in mice via a trans-abdominal approach, clamping renal pedicles via a vascular clip to induce ischemic injury.
Abstract
Acute kidney injury (AKI) is defined as a rapid decline in renal function, in which persistent kidney dysfunction gradually progresses to chronic kidney disease (CKD) due to the irreversible loss of nephrons and their maladaptive repair. In recent years, the incidence of AKI has been increasing concerning diverse etiologies, including volume depletion, sepsis, nephrotoxicity, muscle injury, and major trauma, in which ischemia-reperfusion injury (IRI) accounts for most episodes. Development of the IRI model in mice is induced by surgical clamping of the renal pedicles, which provides powerful and controllable tools for preclinical models of AKI. Importantly, the IRI model is deployed at different stages of the AKI development, especially in the processes of AKI to CKD. Despite the IRI model being widely practiced in many laboratories, a series of variables still influence the results of this model. Here, we describe the procedure of IRI model development to provide a repeatable and reliable method for researchers to explore the underlying pathogenesis in the development of AKI and the progression of AKI to CKD.
Introduction
Acute kidney injury (AKI) is a severe clinical syndrome with significant morbidity and mortality, defined as an increase in serum creatinine of ≥ 0.3 mg/dL (26.5 µM/L) within 48 h or an increase in serum creatinine to ≥ 1.5 times baseline within 7 days, or urine volume < 0.5 mL/kg/h for 6 h1,2,3. Despite decades of research, effective therapy for AKI is lacking to alleviate kidney damage or accelerate kidney recovery, and a considerable proportion of AKI patients progress to chronic kidney disease (CKD)4,5,6. Complex molecules and pathways are involved in AKI and its progression in part, so preclinical models provide powerful tools to unravel these complexities for the development of efficient therapeutic modalities.
Clinically, ischemia-reperfusion injury (IRI) injury is the major cause of AKI in various conditions, including cardiac and hepatic surgeries, circulatory shock, volume depletion, sepsis, renal vascular occlusion or obstruction, kidney transplantation, and so on7. The IRI-AKI mouse model has been in use since the 1960s; this model was developed by surgical clamping of the renal pedicles with non-traumatic clamps in mice leading to ischemia and followed by reperfusion of renal blood flow by removing the clamps. The IRI-AKI model is typically characterized by renal tubular cell death and progressive kidney tissue damage. The IRI is one of the most common models used for the pathogenesis and therapeutic intervention in AKI for several reasons: (1) The simplicity and safety of the surgical procedure improve the survival rate and success rate of the IRI-AKI model8; (2) Since ischemia is a major etiology in human AKI, IRI-AKI model is better used for assessing clinical AKI event9; (3) The IRI model could present kidney injury and histopathology changes in different stages of AKI, which is also applicable to studying the progression from AKI to CKD10. Depending on the experimental design, IRI-induced AKI models include bilateral IRI, unilateral IRI with intact contralateral kidney, and unilateral IRI with simultaneous contralateral nephrectomy. Notably, the bilateral IRI model is considered more relevant to human pathological conditions of AKI because both kidneys have been affected by blood supply11. The IRI model is applicable to simulate the effects of reduced renal blood flow after kidney transplantation, cardiac bypass, renal vascular, or nephron-sparing surgery, as well as in the setting of hypotension9. Here, we describe the procedure for a bilateral IRI model to provide a consistent and reliable method for researchers to explore the underlying pathogenesis in ischemia-induced AKI.
Protocol
Male C57BL/6J mice at 8 weeks of age and weighing 25 g were used to establish the AKI model by bilateral ischemia-reperfusion. As per previous studies, we maintain the body temperature at around 36.5 °C-37 °C, and the kidney ischemia duration is 30 min in the IRI surgery12,13. A total of 6 mice were needed for each group, and sham-operated mice served as controls. The animal experiments in this study have been approved by the Institutional Animal Care and Use Committee (IACUC) of Zhejiang University to protect the welfare of animals. All animal research procedures were performed following the ethical guidelines and principles of Zhejiang University.
1. Pre-operative preparation
- High-pressure sterilize all surgical instruments.
- Prepare the anesthetic solution by adding 2 mL of ketamine and 0.4 mL of xylazine in 7.6 mL of sterile saline. Anesthetize the mouse with a mixture of ketamine (80 mg/kg) and xylazine (16 mg/kg) by intraperitoneal injection. Assess anesthetic depth using the toe pinch reflex.
- Place the anesthetized mouse on the homeothermic blanket to ensure that its airway remains unobstructed. Maintain the body temperature in the range of 36.5-37 °C.
- Cover the mouse's eyes with 1% tetracycline hydrochloride eye ointment to prevent dryness while under anesthesia.
- Shave the hair of the abdomen with a hair clipper and clean the skin of the surgical area with a povidone-iodine solution for 3x.
2. Surgery
- Make an abdominal median incision approximately 1-1.5 cm with surgical scissors via the skin and muscle layer and open the abdominal cavity with a spreader.
- Expose the kidney by moving the retroperitoneal fat and pushing the intestines and other organs to the offside with a cotton swab. The kidney is located in the retroperitoneal space, around 0.5 cm lateral to the spine, and below the 13th rib.
- Dissect the renal pedicle with fine pointed forceps to separate and remove the fascia and adipose tissue and expose the left renal pedicles.
- Clamp the renal pedicles with a vascular clip using holding forceps and ensure that the vascular damage is as minor as possible. Avoid clamping redundant renal sinus fat, which may lead to incomplete renal ischemia.
- Set the kidney ischemia duration, starting with clamping for 30 min. The characteristic of successful ischemia is that the kidney gradually changes from red to deep purple within a few minutes.
- Move the kidney into the retroperitoneal space. Repeat the procedure at the contralateral side to expose and clamp the right renal pedicles.
- Record the ischemia time on each side separately to ensure that both kidneys receive the exact duration of ischemia. Reopen the incision and release the vascular clip at the end of the ischemia duration.
- Replace the kidney into the retroperitoneal space, and then suture the muscle and skin layer by layer with the Vicryl 4-0 suture.
NOTE: The surgical procedures should be performed under sterile conditions. Clean the surgical table and instruments with 75% ethanol during operation when needed.
3. Post-operative care
- Administer 0.5-1 mL of warm and sterile saline by intraperitoneal injection to compensate for the fluid loss.
- Maintain sternal recumbency with attention until the mouse has regained sufficient consciousness. Put the mouse on the homeothermic blanket until it gains full consciousness, then return to its cage. Do not return the mouse to the company of other animals until fully recovered.
- Give buprenorphine 0.05-0.10 mg/kg every 12 h for the first 3 days to alleviate post-surgical pain. Monitor the surgical mice every day.
4. Model assessment
- Hematoxylin-Eosin (HE) staining
- Euthanize animals using pentobarbital sodium by intraperitoneal injection on days 1, 3, 7, or 14 after IRI.
- Fix fresh kidney tissues with 4% paraformaldehyde (PFA) overnight, and store in 75% ethanol at 4 °C.
- After dehydration and embedding, cut the obtained samples into 8 µm thickness for staining.
- Dewax the tissue sections in xylene, and then rehydrate with decreasing concentrations of ethanol.
- Stain the tissue sections with hematoxylin and eosin.
- Dehydrate the tissue sections by increasing concentrations of ethanol and xylene.
- Kidney function assay
- Collect blood samples using the eyeball blood collection method after anesthesia.
- Centrifuge blood samples at 12000 x g for 10 min to separate serum.
- Determine serum creatinine and blood urea nitrogen (BUN) by the automatic dry-chemistry analyzer to monitor renal function.
- Real-time PCR (RT-PCR)
- Extract total RNA from kidney tissues using a quick RNA extraction kit, and then synthesize cDNA with a reverse transcriptase mix kit. Perform RT-PCR with SYBR Green Premix Kit and run on RT-PCR instrument. The primer sequences used here were reported before14: KIM1 forward, 5'-GCTGCTACTGCTCCTTGTGA-3'; reverse 5'-GGAAGGCAACCACGCTTAGA-3'; NGAL forward, 5'-GGCCAGTTCACTCTGGGAAA-3; reverse 5'- TGGCGAACTGGTTGTAGTCC-3'; GAPDH forward, 5'-GGTGAAGGTCGGTGTGAACG-3'; reverse 5'-CTCGCTCCTGGAAGATGGTG-3'.
Results
State of the kidney during surgery
The characteristic of successful ischemia is that the kidney gradually changes from red to deep purple within 1-2 min, and successful reperfusion is characterized by the kidney gradually changing from deep purple to red within 1-2 min.
Histology of the kidney after surgery
HE and Periodic Acid Schiff (PAS) staining are the direct ways to verify acute kidney injury. In the ischemia-reperfusion injury model, the dominant injured site is the S3 segment of proximal tubules. The classic manifestations of renal tubular damage include severe tubular dilation and disruption, loss of brush border, sloughed debris, and cell apoptosis. The tubular damage is also scored by the percentage of damaged tubules in a blinded manner: score 0, no damage; score 1, <25% damaged tubules; score 2, 25%-50% damaged tubules; score 3, 50%-75% damaged tubules; score 4, >75% damaged tubules. We confirmed the characteristic pathological changes of renal tubular and elevated tubular injury score in ischemia-induced AKI (Figure 1A-B).
Function of the kidney after surgery
A decline in kidney function can be assessed by elevated serum creatinine and BUN levels, which were detected by an automatic dry-chemistry analyzer (Figure 1C-D).
Injury of the renal tubule after surgery
Neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (KIM1) are well-established and sensitive biomarkers to evaluate renal tubular dysfunction for the early identification of AKI15. Indeed, we observed that the expression of early tubular injury markers NGAL and KIM1 was elevated in ischemia-induced AKI (Figure1 E).
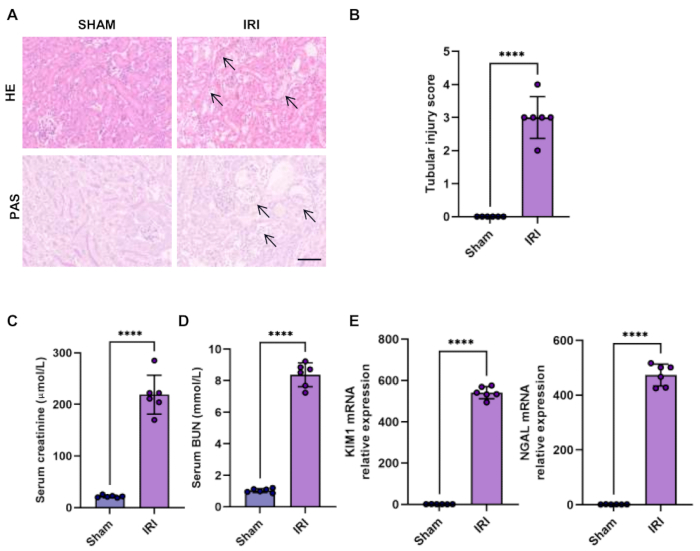
Figure 1: Histology and function of the kidney in the bilateral IRI model. (A-B) Hematoxylin-Eosin staining (HE) and Periodic Acid-Schiff staining (PAS) of renal sections showed kidney injury in the bilateral IRI model (scale bars, 100 µm) and quantification of tubular injury score. Arrows show tubular brush border loss, tubular dilatation and disruption, flattened epithelial cells, and sloughing of tubular epithelial cells. (C-D) Serum creatinine and BUN level of sham and bilateral IRI mouse. (E) RT-qPCR analysis of relative expression of KIM1 and NGAL in the kidney of sham and bilateral IRI mouse. Data were presented as mean ± SD. Statistical analysis was performed using Student's t-test. ****P < 0.0001. Please click here to view a larger version of this figure.
Discussion
In this paper, we have provided a detailed procedure on the renal IRI model, subsequently highlighting that it is a robust model for the progression of AKI and AKI to CKD. In addition, we demonstrate the impact of the two main criteria of kidney injury, including kidney histology and function.
Several key points in surgical procedures need to be emphasized for a repeatable and reliable model. For abdominal surgery, a midline incision is recommended to expose the kidney to minimize trauma associated with the surgery. It is hard to dissect the renal pedicle entirely owing to the retroperitoneal fat, so, peel the renal pedicle from the fat with a pair of forceps via the kidney back and forth. Moreover, it is recommended to apply the same vascular clamp to maintain the stability of the surgical process and pay attention to whether there is wear on the vascular clamp during the procedure. In addition, it is also important to prevent dehydration of mice by giving saline supplementation, owing to the fluid loss that would contribute to kidney injury during surgery.
Several important troubleshooting methods need to be emphasized in the surgical procedures of IRI. First, it is difficult to troubleshoot kidney bleeding while separating the kidney and its pedicle. Most of the bleeding could be controlled by compressing the oozing point adequately, while bleeding volume > 0.5 mL may induce hemorrhagic shock. In this case, euthanize and exclude the mouse from research. Second, incomplete ischemia of the kidney is another aspect to pay attention to. Firstly, incomplete separation of the renal pedicle would lead to lesser ischemic injury. If the renal pedicle is not entirely isolated, the vascular clamp may cause incomplete occlusion, and the operator could change the vascular clamp to confirm complete ischemia of the kidney. Using the same vascular clamp during the procedure and renewing the worn clamp is recommended. Third, renal reperfusion disorder after vascular clamp removal may be due to vascular lesions or blood clot formation, euthanize and exclude such mice from research.
Several critical variables influence the severity of kidney injury in the IRI model. Ischemia time and body temperature are the main determinants of kidney injury. As previous studies reported, tubular injury have been found to be progressive after bilateral kidney ischemia for 25-30 min. Tubular injury aggravates significantly as the ischemic duration increases every 2 min, and an ischemic duration of more than 60 min may result in acute tubular necrosis16. Additionally, core body temperature significantly impacts the outcome in the IRI model. Unsurprisingly, mice with higher body temperatures (36-37 °C) have a more pronounced effect, while no obvious changes have been observed in mice with lower temperatures (33-35 °C) during ischemia8,17. The effect of body temperature on the severity of AKI is connected to metabolism: (1) increasing body temperature results in the damage of cell membranes and the decrease of intracellular energy stores during ischemia; (2) increasing inosine and hypoxanthine levels with higher body temperature produces an increased production of free radicals upon reperfusion18,19. Besides the duration of ischemia and body temperature, several factors should be taken into account for a consistent IRI model of AKI, such as mouse strain, age, gender, body weight, dehydration status, anesthesia, surgery time, and so on20,21. The above variable factors should be considered consistent in the experimental setup.
There are a few limitations in the current IRI-AKI model. First, a well-trained and skillful operator is essential to establish a consistent and reliable model of ischemic AKI, and systematic surgical training should be utilized for technical issues. Further, the severity of kidney injury on histological analysis in mouse IRI model is rarely observed in human AKI22; further exploration of this model is needed to match the state of human AKI. Moreover, mouse strain also affects susceptibility to AKI in the IRI model; it's important to establish IRI-AKI conditions for individual mouse lines.
In conclusion, the bilateral IRI-AKI model is a relative and consistent model for pathogenic investigation and therapeutic approaches. Notably, several critical points in surgical procedures are provided to ensure the transferability of this IRI model. Finally, we highlight that ischemia time and body temperature are the main determinants, and we also mention some additional factors that influence variation within the IRI model.
Disclosures
The authors declare no conflict of interest.
Acknowledgements
We express our appreciation to all participants for their collaboration in the current study. This study was supported by the funding from Zhejiang Provincial Natural Science Foundation of China (LZ22H050001) and Zhejiang provincial program for the Cultivation of High-level Innovative Health talents to Weiqiang Lin.
Materials
| Name | Company | Catalog Number | Comments |
| Animal hair clipper | FEIYUBIO | 19-7002 | |
| 1-ml syringes | Longreen | SR60061 | |
| Ethanol | Macklin | E885996 | |
| Gauze | FEIYUBIO | 19-5022 | |
| Homeothermic monitor system | Warmmate | 40 x 50 | |
| Needle holder | DKBT | CZQ-00160 | |
| Spreader | RWD | R22029-03 | |
| Sterile saline | Biosharp | BL158A | |
| Tissue scissors | DKBT | DC-YKJ1002 | |
| Tissue tweezers | DKBT | DK079904 | |
| Vascular clip | Fine Science Tools | 18055-02 | |
| Vicryl suture | Shanghai Jinhuan | 4 -0 |
References
- Al-Jaghbeer, M., Dealmeida, D., Bilderback, A., Ambrosino, R., Kellum, J. A. Clinical decision support for in-hospital AKI. J Am Soc Nephrol. 29 (2), 654-660 (2018).
- Kellum, J. A., Lameire, N. KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 17 (1), 204 (2013).
- Zhang, C., et al. The Hippo pathway and its correlation with acute kidney injury. Zool Res. 43 (5), 897-910 (2022).
- He, L., et al. AKI on CKD: heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int. 92 (5), 1071-1083 (2017).
- Sato, Y., Takahashi, M., Yanagita, M. Pathophysiology of AKI to CKD progression. Semin Nephrol. 40 (2), 206-215 (2020).
- Wang, Z., Zhang, C. From AKI to CKD: Maladaptive repair and the underlying mechanisms. Int J Mol Sci. 23 (18), 10880 (2022).
- Hoste, E. A. J., et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. 14 (10), 607-625 (2018).
- Wei, Q., Dong, Z. Mouse model of ischemic acute kidney injury: technical notes and tricks. Am J Physiol Renal Physiol. 303 (11), F1487-F1494 (2012).
- Skrypnyk, N. I., Siskind, L. J., Faubel, S., de Caestecker, M. P. Bridging translation for acute kidney injury with better preclinical modeling of human disease. Am J Physiol Renal Physiol. 310 (10), F972-F984 (2016).
- Ferenbach, D. A., Bonventre, J. V. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol. 11 (5), 264-276 (2015).
- Zhang, J., et al. A two-stage bilateral ischemia-reperfusion injury-induced AKI to CKD transition model in mice. Am J Physiol Renal Physiol. 319 (2), F304-F311 (2020).
- Hukriede, N. A., et al. Experimental models of acute kidney injury for translational research. Nat Rev Nephrol. 18 (5), 277-293 (2022).
- Bao, Y., Yuan, Y., Chen, J., Lin, W. Kidney disease models: tools to identify mechanisms and potential therapeutic targets. Zool Res. 39 (2), 72-86 (2018).
- Bao, Y., et al. DNA demethylase Tet2 suppresses cisplatin-induced acute kidney injury. Cell Death Discov. 7, 167 (2021).
- Rossiter, A., La, A., Koyner, J. L., Forni, L. G. New biomarkers in acute kidney injury. Crit Rev Clin Lab Sci. 5, 1-22 (2023).
- Hesketh, E. E., et al. Renal ischemia reperfusion injury: a mouse model of injury and regeneration. J Vis Exp. (88), e51816 (2014).
- Zager, R. A., Altschuld, R. Body temperature: an important determinant of severity of ischemic renal injury. Am J Physiol. 251, F87-F93 (1986).
- Delbridge, M. S., Shrestha, B. M., Raftery, A. T., El Nahas, A. M., Haylor, J. L. The effect of body temperature in a rat model of renal ischemia-reperfusion injury. Transplant Proc. 39 (10), 2983-2985 (2007).
- Le Clef, N., Verhulst, A., D'Haese, P. C., Vervaet, B. A. Unilateral renal ischemia-reperfusion as a robust model for acute to chronic kidney injury in mice. PLoS One. 11 (3), 0152153 (2016).
- Tannenbaum, C., Ellis, R. P., Eyssel, F., Zou, J., Schiebinger, L. Sex and gender analysis improves science and engineering. Nature. 575, 137-146 (2019).
- Lee, H. T., Ota-Setlik, A., Fu, Y., Nasr, S. H., Emala, C. W. Differential protective effects of volatile anesthetics against renal ischemia-reperfusion injury in vivo. Anesthesiology. 101 (6), 1313-1324 (2004).
- Heyman, S. N., Rosen, S., Rosenberger, C. Animal models of renal dysfunction: acute kidney injury. Expert Opin Drug Discov. 4 (6), 629-641 (2009).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved