Method Article
Using Hyperbaric Oxygen to Improve the Radiosensitivity of Human U251 Glioma Cells
In This Article
Summary
This protocol shows that hyperbaric oxygen can enhance the proliferation inhibition and apoptosis of U251 glioma cells treated with X-ray irradiation by blocking the cells in the G2/M phase. This improves the radiosensitivity of human glioma cell lines.
Abstract
The aim of this study was to explore the use of hyperbaric oxygen to enhance the radiosensitivity of human glioma cells. Sub-cultured U251 human glioma cells were randomly divided into four groups: an untreated control group, cells treated with hyperbaric oxygen (HBO) only, cells treated with X-ray irradiation (X-ray) only, and cells treated with both HBO and X-ray. Cell morphology, cell proliferation activity, cell cycle distribution, and apoptosis were observed in these groups to evaluate the role of HBO in improving the radiosensitivity of glioma cells. With the increase in X-ray doses (0 Gy, 2 Gy, 4 Gy, 6 Gy, 8 Gy), the survival fraction (SF) of glioma cells gradually decreased.
Significantly lower SF was observed for the cells treated with the HBO and X-ray together than in the X-ray group for each dose (all P < 0.05). The proliferation inhibition was significantly higher in the HBO combined with X-ray group than in the X-ray group for each dose (all P < 0.05) for the U251 cell line. The percentage of G2/M phase cells was significantly higher in the HBO combined with X-ray (2 Gy) group (26.70% ± 2.46%) and the HBO group (22.36% ± 0.91%) than in the control group (11.56% ± 2.01%) and X-ray (2 Gy) group (10.35% ± 2.69%) (all P < 0.05). U251 cell apoptosis was significantly higher in the HBO combined with X-ray (2 Gy) group than in the HBO group, the X-ray (2 Gy) group, and the control group (all P < 0.05). We conclude that HBO can enhance the proliferation inhibition and apoptosis of glioma U251 cells by blocking glioma cells in the G2/M phase and improve the radiosensitivity of U251 glioma cells.
Introduction
Glioma is a primary intracranial tumor that originates from central nervous system glial cells1. The current treatment strategy for glioma is surgery combined with radiotherapy and chemotherapy. Postoperative radiotherapy for glioma can provide survival benefits (grade I evidence), and early postoperative radiotherapy can effectively prolong patient survival (grade II evidence)2. For higher-grade gliomas (grade III or IV), especially highly malignant and invasive glioblastoma (grade III evidence)3, postoperative radiotherapy should be performed as early as possible (<6 weeks). However, despite early intervention, glioma still has a high recurrence rate and poor prognosis after comprehensive treatment. These outcomes are mainly associated with the low radiosensitivity of glioma. Factors related to tumor radiosensitivity include the inherent radiosensitivity of tumor cells, hypoxic or non-hypoxic tumor cells, the proportion of hypoxic tumor cells, and the capacity of peritumoral tissue to repair radiation damage4.
Among these factors, hypoxic or non-hypoxic tumor cells and the proportion of hypoxic tumor cells have important effects on tumor radiosensitivity. Hyperbaric oxygen (HBO) can improve tissue oxygen storage by increasing tissue oxygen tension and blood oxygen diffusion. HBO may also produce a series of beneficial biochemical, cytological, and physiological effects5. For example, HBO has a marked reparative effect on radiotherapy-induced radiation damage. Although HBO combined with radiotherapy or chemotherapy is reported to improve the clinical efficacy of radiotherapy or chemotherapy for glioma6, there is considerable debate about how HBO alone affects malignant glioma growth. Ding et al.7 and Wang et al.8 both demonstrated that HBO promotes the growth of in situ glioma in mice via mechanisms that involve the inhibition of apoptosis and the promotion of tumor angiogenesis. Under physiological conditions, HBO is reported to promote tumor angiogenesis by inducing oxidative stress9.
However, one study indicated that short-term HBO exposure promotes tumor cell proliferation, whereas prolonged HBO exposure promotes apoptosis and inhibits proliferation10. Therefore, further studies are needed to explore whether HBO promotes or inhibits the growth of glioma and how HBO combined with radiotherapy or chemotherapy can induce therapeutic sensitization. In particular, mechanistic details about how HBO improves the radiosensitivity of glioma are needed. To explore how HBO improves the radiosensitivity of human U251 glioma cells in this study, we used HBO combined with X-ray irradiation on glioma cell proliferation and observed the effects on cell cycle distribution and apoptosis.
Protocol
All study methods were approved by the Institutional Review Board and Ethics Committee of the Second Hospital Affiliated with Lanzhou University and were performed in accordance with relevant guidelines and regulations.
1. Treatment of glioma cells
NOTE: The U251 glioma cell line was used in this experiment.
- U251 cell culture
- Seed U251 cells in multiple dishes with DMEM containing 10% fetal bovine serum (FBS) and culture them at 37 °C with 5% CO2.
- Upon reaching 50%-60% confluency, dissociate the cells using trypsin-EDTA solution (0.25%, without phenol red) and then allow them to grow to 80% confluency.
- X-ray irradiation
- Cover the culture plates or flasks with an equivalent tissue compensator of up to 1 cm thickness. Then, expose the cells to X-ray irradiation delivered from a 6 MV linear accelerator with a source-cell distance of 100 cm, adjusted by clicking the rotate button on the remote control board.
- Have a physicist measure the radiation dose and correct for attenuation. As a control, place flasks containing media in a detector before the X-ray irradiation.
- Hyperbaric oxygen (HBO)
- Disinfect the HBO chamber by turning on the ultraviolet light for 15 min and then flood it with 0.02 MPa of pure oxygen for 5 min.
- After placing the cells in plates or culture flasks in the chamber, click on the pressure regulator button on the control board outside the chamber to increase the HBO pressure in the chamber to 0.2 MPa (2.0 ATA) within 30 min of irradiation.
- Thirty minutes later, click on the pressure regulator button to decrease the HBO pressure to the previous pressure level (0.1 MPpa). Then, treat the culture bottles or plates with HBO 1x per day for 3 consecutive days.
2. U251 glioma cells in different groups
- Set a control group, X-ray (2 Gy) group, and HBO combined with X-ray (2 Gy) group for calculating the cell growth curves.
- Prepare single-cell suspensions from the glioma U251 cells showing the maximum growth rates (see step 1.1.2). Adjust the cell density to 1 × 106/mL by counting the cells using a hemocytometer slide. Subsequently, add 1 mL of the cell suspension to a culture bottle (cell density: 1 × 106/bottle) with three separate bottles for each group.
- Assess cell morphology at 100x magnification with a brightfield microscope to enumerate the adherent cells at 24 h, 48 h, and 72 h of culture.
3. Radiosensitivity of U251 glioma cells (clone formation assay) within 30 min after HBO
- Seed the U251 single-cell suspension throughout the wells at 5 × 102 cells/mL in 6-well plates and then expose them to the indicated dose of X-ray irradiation (0 Gy, 2 Gy, 4 Gy, 6 Gy, and 8 Gy), with three parallel samples being examined for each X-ray dose.
- For the HBO combined with X-ray (2 Gy) group, expose the cells to X-ray irradiation within 30 min of HBO treatment. After treatment, culture the cells at 37 °C with 5% CO2 for 14 days.
- After the clones are visible, remove the medium and wash the clones 2x with PBS.
- Fix the cells in 1 mL of 10% methanol for 15 min before staining with 1 mL of 0.1% crystal violet for 20 min.
- After staining, wash the cells with 6 mL of distilled water using a pipette, and then aspirate the crystal violet solution. Allow the cells to air dry.
- Count the clones with a diameter between 0.3 mm and 1.0 mm under a microscope to ensure that there are >50 cells per clone.
- Calculate the survival fraction (SF) using equation (1):
SF = × 100% (1)
× 100% (1) - Using statistical software, generate a radiation dose-survival curve based on the single-hit multitarget (SHMT) model using equation (2):
S = 1 - (1 -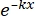 )N (2)
)N (2)
Where S = the probability of survival; k = the mean lethal dose (the dose causing a mean of one hit per cell); x = the number of hits per cell; N = the number of targets (the number of hits for cell death). - Calculate the radiobiological parameters, including the mean lethal dose (D0), quasi-threshold dose (Dq), extrapolation number (N), survival fraction at an irradiation dose of 2 Gy (SF2), sensitization enhancement ratio (D0) (SER = D0 in the control group/D0 in the experimental group), and SER (Dq) (SER = Dq in the control group/Dq in the experimental group) to evaluate the effect of HBO on the radiosensitivity of U251 glioma cells.
Where D0 = the reciprocal value of the slope of the linear portion of the survival curve (the dose reducing the survival rate by 63%)
N = the value of the intersection point formed by extrapolating the linear portion to meet the ordinate (reflecting the cellular ability to repair the radiation-caused damage)
Dq = the value of the intersection-projective point on the abscissa and the intersection formed by drawing a line through 1.0 at the ordinate and parallel to the abscissa to meet the extrapolation line.
4. Cell counting assay to assess U251 glioma cell proliferation
- Set a control group, an HBO group, and groups treated with X-ray (0 Gy, 2 Gy, 4 Gy, 6 Gy, 8 Gy) alone or combined with HBO.
- Adjust the cell density to 1 × 104 cells/mL using U251 cells in single-cell suspensions.
- Seed the cell suspensions (100 µL, density: 1 × 103/well) in 96-well plates (five wells per group). Perform the cell counting assay (see the Table of Materials), and then determine the optical density (OD) at 450 nm using a microplate reader after 48 h of culture with the reagent.
- Calculate the cell proliferation inhibition rate (IR) according to equation (3):
IR = × 100% (3)
× 100% (3)
5. Detection of apoptosis of U251 glioma cells
- Set control, HBO, X-ray (2 Gy), and HBO combined with X-ray (2 Gy) groups.
- Remove the medium after culture and wash the cells with 1x PBS.
- Detach the cells with trypsin and then deactivate the trypsin by adding DMEM when a rounded cell morphology is observed under a microscope.
- Transfer the cells to a centrifuge tube and centrifuge for 5 min at 200 × g.
- Discard the supernatant, add 3 mL of 1x PBS to the pellet, and gently pipette to resuspend the cells.
- Centrifuge the cells again at 200 × g for 5 min. Then, aspirate the supernatant PBS and wash the cells 2x before resuspending them with gentle pipetting in 50 µL of Binding Buffer.
- Add 5 µL of annexin V-FITC to the cells at 4 °C and incubate the cells in the dark at 4 °C for 15 min before adding 400 µL of Binding Buffer. Transfer the mixture to a flow cytometry tube containing 5 µL of propidium iodide (PI) Dye Solution (10 mg/mL). Detect apoptotic cells 5 min later by flow cytometry11.
- Record red fluorescence at the 488 nm excitation wave, input them into a computer to analyze the percentage of each cell cycle in 5,000 cells, and then print the peaks of apoptotic cells.
- Collect red and green fluorescence by annexin V and PI double-labeling, input them into the computer to analyze, and then print the dot plot.
6. Detection of U251 glioma cell cycle distribution
- Wash the above-mentioned cells 2x with 1 mL of PBS before treating them with trypsin.
- When a rounded cell morphology is detected by light microscopy, add DMEM containing 10% FBS.
- Centrifuge the cells for 5 min at 200 × g at room temperature.
- Remove the supernatant and resuspend these cells in 1 mL of PBS before adding precooled 75% ethanol solution.
- Incubate the mixture for at least 4 h or overnight at −20 °C.
- Wash the cells 2x with ice-cold PBS and 180 µL of EDTA (0.1 mM, 3.7 mg of EDTA + 100 mL of PBS), 20 µL of RNase A (10 mg/mL), 35 µL of Triton X-100 (2%, 2 mL of Triton + 98 mL of FBS),and 96.5 µL of PBS. Then, add 17.5 µL of PI solution (1 mg/mL).
- Incubate the mixture at 4 °C in the dark for 10 min.
- Wash the cells in 200 µL of PBS, and then place them in a flow cytometer to assess the cell cycle distribution, as previously described12.
- Operate the flow cytometer in dual-laser, three-dimensional space, excitation mode with a 22 µm x 66 µm and 13 µm x 66 µm spot size. Use a flow room of 430 µm x 180 µm, 300-1,100 nm spectrum, ≤100 MESF detectability, and CV <2% resolution. Then, expose the cells to 488 nm excitation light and detect and measure the fluorescence signals with instrument software to determine the cell cycle distribution13.
- Determine the DNA content, and then according to the DNA content, analyze the cell cycle.
7. Statistical analysis
- Perform statistical analyses.
- Present the data as mean ± standard deviation.
- Use a Student's t-test to compare the groups, with statistical significance set as P < 0.05.
Results
Culture of U251 glioma cells
U251 glioma cells had a fusiform shape 24 h to 48 h after culture in DMEM and were adherent. These cells were used for further study (Figure 1).
Glioma cell morphology and count
The cell counts for the U251 glioma cells in the HBO combined with X-ray (2 Gy) group were significantly lower than that for the X-ray (2 Gy) group after 24 h, 48 h, and 72 h of cell culture (all P < 0.05) (Table 1). When cultured for 24 h, untreated U251 cells in the control group grew well with a long, spindle-shaped morphology. In the X-ray (2 Gy) group, the U251 cell density decreased, with a small number of suspended dead cells visible. In the HBO combined with X-ray (2 Gy) group, the cell density decreased, and there were more suspended dead cells. When cultured for 48 h, the cell density continuously decreased in the X-ray (2 Gy) and the HBO combined with X-ray (2 Gy) groups, while the density increased for the control group. When cultured for 72 h, the cell density further decreased in the X-ray (2 Gy) group, particularly the HBO combined with X-ray (2 Gy) group (Figure 2).
Effects of HBO treatment on the radiosensitivity of U251 glioma cells
The SF decreased dose dependently with increasing radiation dose (0 Gy, 2 Gy, 4 Gy, 6 Gy, and 8 Gy) in the X-ray and HBO combined with X-ray groups. The SF was significantly lower in the HBO combined with X-ray group compared to the X-ray group for all radiation doses tested (all P < 0.05) (Table 2). Together, these results show that HBO treatment increased the radiosensitivity of the U251 glioma cells.
D0, Dq, N, and SF2 were inversely associated with radiosensitivity and were lower in the HBO combined with X-ray group than in the X-ray group for the U251 cells. These results demonstrate that HBO increased the radiosensitivity of the U251 glioma cells (Table 3).
Effects of HBO treatment on radiation-induced inhibition of U251 glioma cell proliferation
With increasing radiation dose (0 Gy, 2 Gy, 4 Gy, 6 Gy, and 8 Gy), the inhibition of the cell proliferation gradually increased for both the X-ray groups and those that received HBO combined with X-ray. The proliferation rate was significantly inhibited for the HBO combined with X-ray group compared to the X-ray-only group at all radiation doses tested (all P < 0.05). These results indicate that HBO enhanced the inhibitory effect of X-ray radiation on the proliferation of U251 glioma cells (Table 4).
Effect of HBO on U251 glioma cell cycle distribution and apoptosis
The percentage of cells in the G2/M phase of the cell cycle was significantly higher for the HBO combined with X-ray (2 Gy) group (26.70% ± 2.46%) and HBO group (22.36% ± 0.91%) compared to the control group (11.56% ± 2.01%) and X-ray (2 Gy) group (10.35% ± 2.69%) (all P < 0.05). However, there were no significant differences in the percentage of G2/M phase cells between the control group and the X-ray (2 Gy)-only group or between the HBO group and the HBO combined with X-ray (2 Gy) group (all P > 0.05) (Table 5, Figure 3, and Figure 4). Thus, HBO likely arrested U251 glioma cells in the G2/M phase to promote glioma U251 cell cycle synchronization.
The number of apoptotic U251 cells was significantly higher in the HBO and X-ray (2 Gy) groups than the control group (all P < 0.05) and in the HBO combined with X-ray (2 Gy) group compared to the HBO, X-ray (2 Gy), and control groups (all P < 0.05) (Figure 5 and Table 5). From the above results, both HBO and X-ray appeared to induce U251 cell apoptosis, but the ability of HBO combined with X-ray to induce apoptosis of U251 cells was more pronounced.
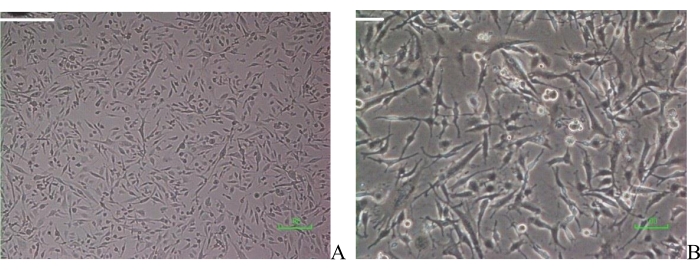
Figure 1: Glioma U251 cell morphology. (A) U251 cells (20x); (B) glioma U251 cells (100x). Scale bar = 10 µm. Please click here to view a larger version of this figure.

Figure 2: Morphology of cells in the different groups 24 h, 48 h, and 72 h after cell culture. Control group, 20x; HBO combined with X-ray, 20x; X-ray (2 Gy), 20x; HBO group, 100x. Abbreviations: HBO = hyperbaric oxygen. Scale bar = 10 µm. Please click here to view a larger version of this figure.

Figure 3: Glioma U251 cell cycle distribution in each group (n = 3). Abbreviation: HBO = hyperbaric oxygen. Please click here to view a larger version of this figure.
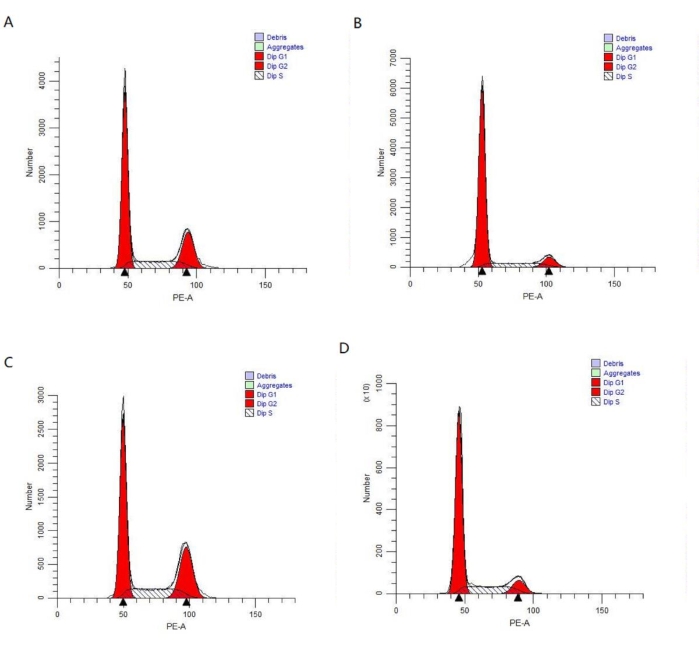
Figure 4: Glioma U251 cell cycle distribution in each group (n = 3). (A) HBO group; (B) X-ray (2 Gy) group; (C) HBO combined with X-ray (2 Gy) group; (D) Control group. Abbreviations: HBO = hyperbaric oxygen; PE-A = phycoerythrin-peak area. Please click here to view a larger version of this figure.
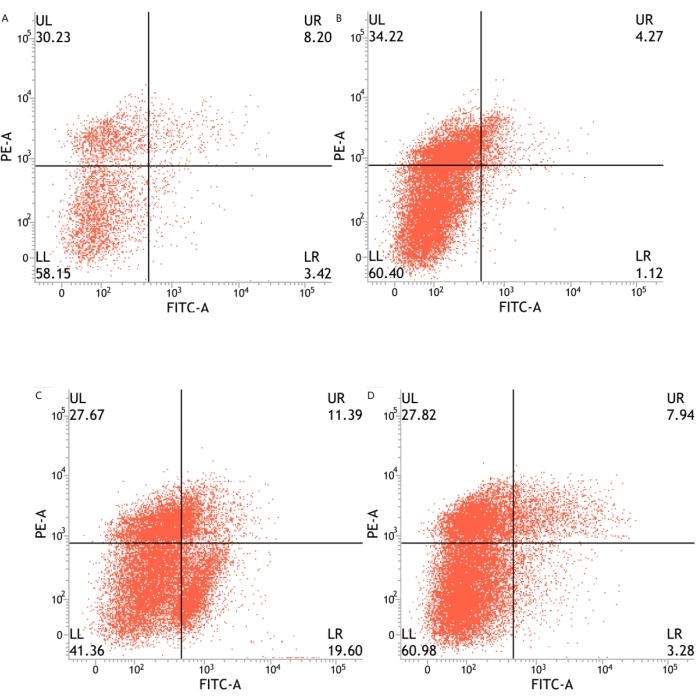
Figure 5: U251 glioma cell apoptosis in each group (n = 3). (A) X-ray (2 Gy) group; (B) Control group; (C) HBO combined with X-ray (2 Gy) group; (D) HBO group. Abbreviations: HBO = hyperbaric oxygen; PE-A = phycoerythrin-peak area; FITC-A = fluorescein isothiocyanate-peak area. Please click here to view a larger version of this figure.
| (x̄ ± s) (n=3) × 106 | |||
| Groups | 24 h | 48 h | 72 h |
| Control group | 2.03±0.17 | 3.27±0.21 | 5.94±0.16 |
| HBO combined with X-ray | 1.04±0.13 | 0.36±0.06 | 0.08±0.04 |
| X-ray | 1.94±0.09 | 0.79±0.09 | 0.43±0.10 |
| HBO | 2.34±0.10 | 3.50±0.12 | 6.00±0.15 |
| P | 0.017 | 0.03 | 0.013 |
Table 1: U251 cell count for each group. Cell counts are 1 × 106. P is for HBO combined with X-ray (2 Gy) versus X-ray (2 Gy). Abbreviation: HBO = hyperbaric oxygen.
| (x̄ ± s) (n=3) | |||||
| Groups | 0 Gy | 2 Gy | 4 Gy | 6 Gy | 8 Gy |
| X-ray | 1 | 0.66 | 0.424 | 0.301 | 0.075 |
| HBO combined with X-ray | 0.75 | 0.319 | 0.235 | 0.109 | 0.019 |
| P | 0.019 | 0.019 | 0.031 | 0.04 | 0.036 |
Table 2: U251 cell survival fraction for each group. Abbreviation: HBO = hyperbaric oxygen.
| (n=3) | ||||||
| Items | D0 (Gy) | Dq (Gy) | N | SF2 | SER (D0) | SER (Dq) |
| Groups | ||||||
| X-ray | 4.01 | 2.305 | 1.314 | 0.66 | ---- ---- | ---- ---- |
| HBO combined with X-ray | 2.64 | 1.143 | 0.436 | 0.319 | 1.52 | 2.02 |
Table 3: Radiobiological parameters of U251 cells in the X-ray and HBO combined with X-ray groups. n = 3. Abbreviations: HBO = hyperbaric oxygen; D0 = mean lethal dose; Dq = quasi-threshold dose; N = extrapolation number; SF2 = survival fraction at an irradiation dose of 2 Gy; SER = sensitization enhancement ratio; SER (D0) = D0 in the control group/D0 in the experimental group; SER (Dq) = Dq in the control group/Dq in the experimental group.
| (n=5) | |||||
| Groups | 0 Gy | 2 Gy | 4 Gy | 6 Gy | 8 Gy |
| X-ray | 0 | 0.1026 | 0.153 | 0.2157 | 0.2327 |
| HBO combined with X-ray | 0.0189 | 0.2039 | 0.2622 | 0.3143 | 0.336 |
| P | 0.045 | 0.018 | 0.026 | 0.02 | 0.015 |
Table 4: Inhibition of proliferation of U251 glioma cells in each group. n = 5. Abbreviation: HBO = hyperbaric oxygen.
| Cycles | G1/G0 | S | G2/M | Apoptosis |
| Groups | ||||
| HBO | 59.23±1.46 | 18.41±0.54 | 22.36±0.91*# | 10.25±4.48*& |
| X-ray | 74.77±4.14 | 14.89±1.45* | 10.35±2.69 | 12.31±5.39*& |
| HBO combined with X-ray | 53.60±3.23 | 19.72±0.76 | 26.70±2.46*# | 28.89±8.78* |
| Control | 67.96±2.41 | 20.49±0.40 | 11.56±2.01 | 5.21±2.03& |
Table 5: Cell cycle distribution and apoptosis of U251 cells in each group. n = 3, %. Abbreviations: HBO = hyperbaric oxygen. * indicates P < 0.05 as compared with the control group. # indicates P < 0.05 as compared with the X-ray group. & indicates P < 0.05 as compared with HBO combined with X-ray.
Discussion
The glioma cell line U251 is one of the most classical human glioma cell lines and is widely used as a glioma model in many studies.
Effects of HBO on U251 glioma cell proliferation
HBO typically refers to breathing pure oxygen (100% oxygen concentration) in a sealed chamber with a pressure 1.5-3-fold higher than normal atmospheric pressure, which can increase oxygen content in microvascular plasma14. For patients with glioma, combining radiotherapy or chemotherapy with HBO is reported to increase the clinical efficacy of these treatments. In children or young adults with central nervous system neoplasms, HBO combined with radiotherapy can improve clinical symptoms and tumor imaging results. Although the application of HBO is safe6, there is considerable debate about the effects of HBO alone on malignant glioma growth. Ding et al.7 showed that HBO inhibited apoptosis and increased microvascular density in tumor tissues by promoting the expression of vascular endothelial growth factor and hypoxia-inducible factor-1a, suggesting that pure HBO would not be beneficial for the treatment of glioma. HBO was reported to promote glioma cell growth in a rat model of glioma8 and promote tumor angiogenesis by inducing oxidative stress under physiological conditions9. Short-term exposure to HBO promotes tumor cell proliferation, whereas prolonged exposure inhibits tumor cell proliferation10. HBO inhibits apoptosis and reduces tumor microvessel density in subcutaneously transplanted gliomas; however, these effects are not seen in intracranial transplanted glioma15,16. This study showed that the inhibition of cell proliferation rate was significantly higher for the HBO combined with X-ray group than for the X-ray group at each dose (all P < 0.05), as well as for the HBO group compared to the control group (P < 0.05). This result suggests that HBO can enhance the inhibitory effect of X-rays on U251 glioma cell proliferation and that HBO alone does not promote the proliferation of these glioma cells. The effect of HBO on tumor growth may be associated with a variety of factors, such as tumor site and differentiation status, as well as HBO pressure and HBO duration. In this study, HBO at 0.2 MPa (2.0 ATA) was used for 90 min, and the HBO treatment was repeated three times. The relationships of HBO duration, frequency, and pressure with U251 glioma cell proliferation will require further investigation. These results indicated that, with increasing radiation dose (0 Gy, 2 Gy, 4 Gy, 6 Gy, and 8 Gy), the inhibition of cell proliferation gradually increased in the X-ray groups and HBO combined with X-ray groups, and the proliferation rate was significantly lower for the HBO combined with X-ray group than for the X-ray group for each radiation dose. This result demonstrates that HBO can enhance the inhibitory effect of X-rays on the proliferation of U251 glioma cells.
Radiotherapy can produce reactive oxygen species (ROS) that cause DNA damage in tumor cells. However, hypoxia in tumor tissues directly reduces ROS production, which can decrease the therapeutic effect of radiotherapy on tumor cells. In the anoxic microenvironment, tumor cells are likely to bind to hydrogen atoms from sulfhydryl compounds, and this binding neutralizes the free radicals generated by ionizing radiation and decreases tumor cell DNA injury. However, in an oxygen-enriched environment, the free radicals generated by ionizing radiation are rapidly oxidized and capable of damaging tumor cell DNA. This may be the potential mechanism by which HBO can enhance the inhibitory effect of X-rays on U251 glioma cell proliferation.
The effect of HBO on U251 glioma cell radiosensitivity
A clone formation assay is simple and suitable for adherent cells. The preparation of cell suspensions and the inoculation density are important for this assay. The cells must be well dispersed without cell clusters, and the inoculation density should not be too high. The limitation of this clone formation assay is that only adherent cells can be investigated for the effects of HBO on proliferation.
In this study, the SF was significantly lower for the HBO group (0.750 ± 0.023) than the control group (1.000 ± 0.000) (P = 0.019), demonstrating that HBO does not promote U251 glioma cell growth. This result is consistent with those for the cell counting assay. With increasing radiation dose (0 Gy, 2 Gy, 4 Gy, 6 Gy, and 8 Gy), the SF gradually decreased and was significantly lower for the HBO combined with X-ray group than for the X-ray group at each radiation dose (all P < 0.05), demonstrating that the radiosensitivity of U251 glioma cells increased after HBO treatment. In addition, the survival curves for the U251 glioma cells, as well as all the radiobiological parameters, indicated that SF2, D0, and Dq were significantly lower for the HBO combined with X-ray group than for the X-ray group, suggesting that HBO can improve the radiosensitivity of glioma cells. SF2 is reported to be closely related to the post-radiotherapy 2 year tumor survival rate, local control rate, and recurrence rate17,18,19.
The effects of HBO on the U251 glioma cell cycle and apoptosis
DNA characteristics vary among the phases of the cell cycle. For example, cellular DNA is 2N in the G0/G1 phase and 4N in the G2/M phase. Propidium iodide (PI) can bind to DNA, and the fluorescence intensity of the complex directly reflects the intracellular DNA status. Therefore, flow cytometry and PI staining can be used to detect the cell cycle. The key to this experimental technology is an increase in the permeability of the cellular membranes that reduces cell adhesion. In cells treated with ethanol, Triton X-100, and EDTA, the final concentration of PI must reach 50 µg/mL to allow for accurate detection of the cell cycle distribution.
The cell cycle comprises G0/G1, S, and G2/M phases, which play an important role in regulating tumor cell growth. The G0/G1 phase is the preparatory stage of DNA synthesis or the initial stage of cell proliferation, whereas the S phase is the active stage of cell proliferation in which DNA replication is completed. The G2/M phase is the stage in which genetic material is distributed and new daughter cells form. Any factor that blocks a particular phase of the cell cycle can induce cellular synchronization and, in turn, a relative increase in the proportion of cells in a certain phase20. Cells in different cell cycle phases have different radiosensitivity. For example, cells in early G1 phase exhibit radioantagonism, whereas cells in the G1/S phase have slightly elevated radiosensitivity. Cells in the S phase have a moderate elevation of radiosensitivity, and G2/M phase cells have the highest radiosensitivity. This study showed that the percentage of G2/M phase cells was significantly higher in the HBO combined with X-ray group than in the X-ray and control groups. However, there was no significant difference in the percentage of G2/M phase cells between the HBO and HBO combined with X-ray groups, suggesting that HBO can cause U251 glioma cells to arrest in the G2/M phase. This conclusion is consistent with the findings reported by Kalns and Piepmeier21. Therefore, cell cycle synchronization in the G2/M phase not only inhibits tumor cell proliferation but also increases the sensitivity of tumor cells to radiotherapy.
Apoptosis plays an important role in human embryo development, tissue repair, and internal environment stabilization. This study showed that the apoptosis of U251 cells was significantly higher for the HBO and X-ray groups than the control group, as well as for the HBO combined with X-ray group compared to the HBO, X-ray, and control groups, suggesting that HBO and X-ray together may induce U251 cell apoptosis and that HBO can enhance X-ray-induced apoptosis. Apoptosis is closely related to tumor growth and development. X-ray and HBO are both reported to induce tumor cell apoptosis10,15,16, which is consistent with the results of this study. HBO may promote apoptosis by regulating the expression of apoptosis-related genes such as aspartate protein hydrolase (caspase), B-cell lymphoma-2 (Bcl-2), and B-lymphoma (Bax)22. The Bcl-2 gene family proteins play an important role in regulating tumor radiosensitivity. For example, increased Bcl-2 gene expression can antagonize radiation-induced apoptosis, while Bax gene overexpression can enhance tumor radiosensitivity23,24. HBO can improve tumor radiosensitivity by downregulating Bcl-2 and Bcl-xl expression while upregulating that of Bax25,26.
In summary, HBO may be used to treat glioma because it can enhance the apoptosis, proliferation inhibition, and radiosensitivity of U251 glioma cells by blocking the cells in the G2/M phase. However, further study is needed to characterize in detail the molecular mechanisms involved in the ability of HBO to enhance glioma cell radiosensitivity and to induce apoptosis.
Disclosures
The authors have no conflicts of interest to declare.
Acknowledgements
None.
Materials
| Name | Company | Catalog Number | Comments |
| Binding Buffer | Dickinson and Company | RH10 9RR | |
| CCK-8 test kit | DOJINDO | NJ | Cell counting assay |
| CELL FIT | cell cycle analysis (DNA content) | ||
| CELLQUEST | apoptotic cell analysis | ||
| DMEM and Annexin V-FITC | Gibco BRL | ||
| flow cytometer | Dickinson | ||
| Glioma U251 and U87 cell line | Shanghai Institute of Cell Biology | ||
| hyperbaric oxygen chamber | Hongyuan Institute | ||
| medical linear accelerator | Elekta Limited Company | ||
| microplate reader | |||
| MOD FITLT formac v1.01 | cell analysis--cell cycle phase | ||
| trypsin | Hyclone Laboratories Inc |
References
- Louis, D. N., et al. The 2016 World Health Organization Classification the Central Nervous System: A summary. Acta Neuropathologica. 131 (6), 803-820 (2016).
- Sun, M. Z., et al. Survival impact of time to initiation of chemoradiotherapy after resection of newly diagnosed glioblastoma. Journal of Neurosurgery. 122 (5), 1144-1150 (2015).
- Hegi, M. E., et al. MGMT gene silencing and benefit from temoozolomide in glioblastoma. New England Journal of Medicine. 352 (10), 997-1003 (2005).
- Zhu, Y., et al. Involvement of decreased hypoxia-inducible factor 1 activity and resultant G1-S cell cycle transition in radioresistance of perinecrotic tumor cells. Oncogene. 32 (16), 2058-2068 (2013).
- Kohshi, K., et al. Potential roles of hyperbaric oxygenation in the treatments of brain tumors. Undersea and Hyperbaric Medicine. 40 (4), 351-362 (2013).
- Aghajan, Y., Grover, I., Gorsi, H., Tumblin, M., Crawford, J. R. Use of hyperbaric oxygen therapy in pediatric neuro-oncology: a single institutional experience. Journal Neurooncology. 141 (1), 151-158 (2019).
- Ding, J. B., Chen, J. R., Xu, H. Z., Qin, Z. Y. Effect of hyperbaric oxygen on the growth of intracranial glioma in rats. Chinese Medical Journal. 128 (23), 3197-3203 (2015).
- Wang, Y. G., et al. Hyperbaric oxygen promotes malignant glioma cell growth and inhibits cell apoptosis. Oncology Letters. 10 (1), 189-195 (2015).
- Milovanova, T. N., et al. Hyperbaric oxygen stimulates vasculogenic stem cell growth and differentiation in vivo. Journal of Applied Physiology. 106 (2), 711-728 (2009).
- Conconi, M. T., et al. Effects of hyperbaric oxygen on proliferative and apoptotic activities and reactive oxygen species generation in mouse fibroblast 3T3/J2 cell line. Journal of Investigative Medicine. 51 (4), 227-232 (2003).
- Vermes, I., Haanen, C., Steffiens-Nakken, H., Reutellingsperger, C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. Journal of Immunological Methods. 184 (1), 39-51 (1995).
- Cui, W., Niu, F. -. L., He, L. -. Y., W, S. -. R. Comparison of two softwares for analysing apoptosis with flow cytometry. Journal of Beijing University of Traditional Chinese Medicine. 24 (6), 45-47 (2001).
- Cecchini, M. J., Amiri, M., Dick, F. A. Analysis of cell cycle position in mammalian cells. Journal of Visualized Experiments. (59), e3491 (2012).
- Resanovic, I., et al. Effects of hyperbaric oxygen on inducible nitric oxide synthase activity/expression in lymphocytes of type 1 diabetes patients: A prospective pilot study. International Journal of Endocrinology. 2019, 2328505 (2019).
- Stuhr, L. E., et al. Hyperoxia retards growth and induces apoptosis, changes in vascular density and gene expression in transplanted gliomas in nude rats. Journal of Neuro-Oncology. 85 (2), 191-202 (2007).
- Biollaz, G., et al. Site-specific anti-tumor immunity: differences in DC function, TGF-beta production and numbers of intratumoral Foxp3+ Treg. European Journal of Immunology. 39 (5), 1323-1333 (2009).
- McKenna, F. W., Ahmad, S. Fitting techniques of cell survival curves in high-dose region for use in stereotactic body radiation therapy. Physics in Medicine and Biology. 54 (6), 1593-1608 (2009).
- Malaise, E. P., Lambin, P., Joiner, M. C. Radiosensitivity of human cell lines to small doses. Are there some clinical implications. Radiation Research. 138, S25-S27 (1994).
- Björk-Eriksson, T., West, C., Karlsson, E., Mercke, C. Tumor radiosensitivity (SF2) is a prognostic factor for local control in head and neck cancers. International Journal of Radiation Oncology Biology Physics. 46 (1), 13-19 (2000).
- Bromfield, G. P., Meng, A., Warde, P., Bristow, R. G. Cell death in irradiated prostate epithelial cells: Role of apoptotic and clonogenic cell kill. Prostate Cancer and Prostatic Diseases. 6 (1), 73-85 (2003).
- Kalns, J. E., Piepmeier, E. H. Exposure to hyperbaric oxygen induces cell cycle perturbation in prostate cancer cells. In Vitro Cellular & Developmental Biology - Animal. 35 (2), 98-101 (1999).
- Lakka, S. S., et al. Inhibition of cathepsin B and MMP-9 gene expression in glioblastoma cell line via RNA interference reduces tumor cell invasion, tumor growth and angiogenesis. Oncogene. 23 (27), 4681-4689 (2004).
- Li, S., Shi, D., Zhang, L., Yang, F., Cheng, G. Oridonin enhances the radiosensitivity of lung cancer cells by upregulating Bax and downregulating Bcl-2. Experimental and Therapeutic Medicine. 16 (6), 4859-4864 (2018).
- Campbell, K. J., Tait, S. W. G. Targeting BCL-2 regulated apoptosis in cancer. Open Biology. 8 (5), 180002 (2018).
- Rengarajan, T., et al. D-pinitol promotes apoptosis in MCF-7 cells via induction of p53 and Bax and inhibition of Bcl-2 and NF-κB. Asian Pacific Journal of Cancer Prevention. 15 (4), 1757-1762 (2014).
- Shinagawa, A., et al. The potent peptide antagonist to angiogenesis, C16Y and cisplatin act synergistically in the down-regulation of the Bcl-2/Bax ratio and the induction of apoptosis in human ovarian cancer cells. International Journal of Radiation Oncology Biology Physics. 39 (6), 135-164 (2011).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved