Method Article
Establishment of an Ex Vivo Lung Perfusion Rat Model for Translational Insights in Lung Transplantation
In This Article
Summary
Here, we describe the necessary steps for establishing a rat EVLP model and show the inflammatory profile associated with the perfused lungs. The aim is to propagate knowledge and experiences about the rat EVLP model, enabling the integral understanding of the biological responses associated with that revolutionary technique.
Abstract
Since the establishment of lung transplantation as a therapeutic strategy for advanced lung diseases, the scientific community is faced with the problem of a low number of lungs considered viable for the donation process. In recent decades, however, this scenario has been positively changed, given the development of ex vivo lung perfusion (EVLP) as a strategy for evaluating and reconditioning marginal lungs. The establishment of EVLP in large transplant centers has favored an increase in the number of lung transplants, both by increasing the diagnostic accuracy of lung function and by constituting an effective platform for the reconditioning of lung grafts. In this context, faced with ethical and logistical issues, as well as in the study of immunological factors associated with lung transplantation, the development of rodent EVLP models has become important, given their reliability, the possibility of genetic manipulation, and lower costs. This paper describes a protocol for establishing a rat EVLP model and shows the inflammatory profile associated with the perfused lungs. This will help propagate knowledge about the rat EVLP model, promoting our understanding of the biological responses associated with that revolutionary technique.
Introduction
Lung transplantation has been recognized as a therapeutic strategy for end-stage diseases with improvements in surgical methods and immunosuppression during the last decades. Despite the high demand, the number of acceptable deceased lung donors is lower than for other solid organs, converging to the lower number of lung transplants performed1,2,3. To address the donor pool shortage, the medical community has expanded the criteria for lung donation, turning organs previously considered nonviable into potential organs for transplantation. However, the extended criteria require different efforts for a better understanding and intervention in view of the possible systemic consequences originating from the donated organ. Ex vivo lung perfusion (EVLP) emerged as a technique that provides normothermic lung preservation, assessment of lung functions, and reconditioning of lungs previously considered unfeasible for the donation process4,5,6.
Given the growing number of lung transplants since the establishment of EVLP in large transplant centers, lung preservation and repair strategies have been increasingly investigated. In this sense, faced with ethical and logistical issues, as well as in the study of immunological factors associated with lung transplantation, the development of rodent EVLP models has become important, given their reliability, the possibility of genetic manipulation, and lower costs7,8,9. Here, we describe the necessary steps to establish a rat EVLP model and show the inflammatory profile associated with the perfused lungs.
Protocol
Animal experiments were performed in compliance with the Animal Use Protocol approved by the Animal Care Committee at the University Health Network. Male Lewis rats (255-330 g) were given ad libitum access to food and water. Then, they were maintained in a controlled environment (18-22 °C) with a 12 h day-night cycle. See the Table of Materials for details related to all materials, solutions, and instruments used in this protocol.
1. Initialization of the ex vivo lung perfusion system
- Ensure that all transducers are connected to the isolated perfusion organ equipment and the data acquisition system (Figure 1). Then, open the data acquisition software.
- Before perfusion, fill the EVLP circuit with 150 mL of Steen solution supplemented with 1,000 USP units of sodium heparin, 50 mg of cefazolin, and 50 mg of methylprednisolone.
- Set the warm water bath to 20 °C. Start circulating warm water to warm up the entire EVLP system.
2. Donor lung procurement procedure
- Anesthetize the rat with an intraperitoneal injection of ketamine (50 mg/kg) and xylazine (5 mg/kg) or according to local practice. Confirm proper depth of anesthesia by checking the toe pinch reflex.
- Perform orotracheal intubation (14 G intravenous catheter). After intubation, connect the tracheal tube to the small-animal ventilation system. Ventilate the rat with a tidal volume of 10 mL/kg, rate of 60 breaths/min, inspired oxygen fraction (FiO2) of 0.5, and positive end expiratory pressure (PEEP) of 2 cmH2O.
- Place the rat in the supine position.
- With the help of appropriate scissors and forceps, enter the peritoneal cavity and carry the incision cranially, performing a median laparotomy and injecting sodium heparin (400 USP units) into the portal vein.
- Subsequently, enter the thoracic cavity through the xyphoid process. Carry out the sternotomy with the cranial resection of the sternum and cautious radial opening of the diaphragm, so as not to damage the lung.
- To retrieve the heart and lung block, make an incision in the inferior vena cava (IVC) and along the apex of the left heart.
- Using micro scissors, perform an anterior incision in the right ventricular outflow tract. Then, insert an 18 G intravenous catheter into the pulmonary trunk and flush the lungs with 20 mL of low potassium dextran (LPD) solution containing 10 µg/mL of prostaglandin E1.
- Immediately after flushing, clamp the lower third of the trachea at the end of inspiration to preserve the lungs in an inflated state.
- Harvest the heart and lung block and place it in LPD solution for storage.
NOTE: The lung graft can be stored at temperatures and different intervals of time according to the research purpose. Here, we show representative results of lungs that underwent varying lengths of cold ischemic time (CIT) on ice.
3. Ex vivo lung perfusion procedure
- Place a 0 silk ligature beneath the main pulmonary artery (PA) and around the ventricles, and pretied to facilitate cannulation.
- Connect the PA cannula in the inflow line of the EVLP system and start the peristaltic pump with 10% of maintenance flow, allowing the removal of any air in the PA cannula.
- Place and secure the inflow cannula into the main PA (OD: 2.0 mm/Head Diameter: 2.5 mm), followed by the drainage cannula from the apex of the heart into the left atrium (LA-Head Diameter: 4.0 mm) through the mitral valve for subsequent perfusion. Use forceps to dilate the mitral valve, facilitating the cannulation.
- Make a small hole in the trachea and insert the tracheal cannula (OD: 2.0 mm/L: 14 mm). Then, connect it to the ventilation line of the system.
- Connect the LA cannula to the outflow line of the system.
- NOTE: We set 10% of maintenance flow as the initiation of perfusion. To avoid the insertion of air bubbles in the lungs, ensure the bubble trap is filled.
- Gradually increase the flow rate perfusion to reach 20% of the cardiac output (CO).
NOTE: To perfuse both lungs, we used an estimated CO of 75 mL/min for 250 g rats10,11. The flow rate of perfusion was gradually increased to 1 h to reach the target flow rate according to our clinical EVLP protocol12. The perfusion parameters are summarized in Table 113. - Remove the tracheal clamp 20 min after the initiation of perfusion and start lung ventilation followed by starting the flow of EVLP gas (8% CO2, 6% O2, 86% N2) to maintain inflow perfusate PCO2 of between 35 and 45 mmHg.
NOTE: The ventilation parameters are summarized in Table 113.
4. Parameters and sample management
- Every hour during EVLP, record in real time the dynamic lung compliance and pulmonary vascular resistance (PVR).
NOTE: At 5 min before the physiological assessments, the lungs should be expanded to an inflation pressure. - Take perfusate samples from the sample port every hour and flash freeze in liquid nitrogen for further analyses.
NOTE: In addition, we perform a recruitment maneuver (up to 20 cmH2O) 5 min before assessment, after which we take perfusates for blood gas analyses to measure pH, PCO2, PO2, electrolytes, glucose, and lactate. - Stop perfusion and clamp the trachea to maintain the lungs in an inflated state. Subsequently, isolate lung samples and flash freeze them in liquid nitrogen or place them in fixing solutions for further studies.
NOTE: Here, the lungs were perfused for 4 h.
Results
All lungs with CIT ranging from 20 min to 18 h could be perfused for 4 h (Figure 2)13. Compliance was stable in most groups with the exception of 18 h CIT, which gradually decreased over the 4 h perfusion period (Figure 2A). Despite this, no significant differences in vascular resistance, lung graft oxygenation, and glucose levels were observed for the groups (Figure 2B-D). Lactate increased over time, was different between the groups, and tended to be higher with longer CIT (Figure 2E). Perfusate electrolytes were similar (Figure 2F-H). At the end of EVLP lung, the level of edema formation was not significantly different among groups with up to 18 h CIT; 24 h CIT led to quite severe edema13. Representative lung appearance at the end of EVLP is shown in Figure 2J. Lung tissue and perfusate inflammatory profile after 4 h of EVLP are shown in Table 213.
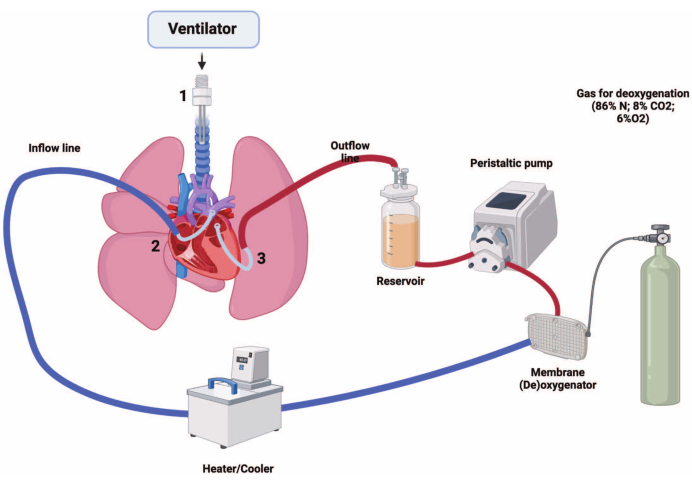
Figure 1: Rat ex vivo lung perfusion system design. 1, Tracheal cannula; 2, pulmonary artery cannula; 3, left atrium cannula. Please click here to view a larger version of this figure.

Figure 2: Lung function during rat EVLP protocol. (A-I) Lung function and blood gas analysis of donor lungs undergoing EVLP after exposure to different CITs. Each CIT group is depicted in a different color: 20 min (20 min-CIT) in red, 6 h (6 h-CIT) in green, 12 h (12 h-CIT) in blue, and 18 h (18 h-CIT) in black. (J) Rat donor lung appearance after 4-h EVLP in each CIT group. This figure was reproduced from Ohsumi et al.13 with permission. Abbreviations: EVLP = ex vivo lung perfusion; CIT = cold ischemic time; W/D, wet-to-dry weight. Please click here to view a larger version of this figure.
| Technique | Parameters | Details | |
| Perfusion | Perfusion Flow | 20% of estimated CO (estimated CO: 75 ml/kg for 250 g rats) | |
| (Constant flow perfusion) | Perfusate | 150 mL of Steen solution with 1.000 USP units of sodium heparin and 50 mg of methylprednisolone | |
| LA pressure | 3 cmH2O | ||
| Ventilation | Inspiratory/expiratory pressure | 10/5 cmH2O | |
| (Pressure control ventilation) | Respiratory rate | 40 breaths/min | |
| FiO2 | Room air | ||
| Recruitment maneuver | Up to 20 cmH2O - 5 min before assessment, every 30 min | ||
| Deoxygenation | Mixed gas | 6% O2, 8% CO2 and balanced N2 | |
| (Using a gas exchange membrane) | |||
Table 1: Rat EVLP perfusion strategy. Abbreviations: EVLP, ex vivo lung perfusion system; LA, left atrium; CO, cardiac output.
| Cytokine/Group | 20 min - CIT (n = 4) | 6 h – CIT (n = 5) | 12 h – CIT (n = 5) | 18 h – CIT (n = 5) | P-Value | |
| Interleukin-1β | ||||||
| Perfusate after 4 h EVLP (pg/mL) | 4.85 ± 1.56 | 3.40 ± 2.36 | 3.55 ± 2.15 | 3.36 ± 1.67 | 0.56 | |
| Lung tissue after 4 h EVLP (pg/mg) | 74.8 ± 61.6 | 110.3 ± 31.1 | 97.1 ± 61.6 | 165.2 ± 31.2 | 0.12 | |
| Interleukin-6 | ||||||
| Perfusate after 4h EVLP (pg/mL) | 297.1 ± 75.9 | 100.5 ± 89.2 | 247.0 ± 138.7 | 311.6 ± 237.6 | 0.11 | |
| Lung tissue after 4h EVLP (pg/mg) | 9.1 ± 18.1 | 7.3 ± 16.2 | 80.2 ± 179.3 | 137.1 ± 84.9 | 0.12 | |
Table 2: Inflammatory profile at the end of rat EVLP protocol. This table was adapted from Ohsumi et al.13 with permission. Data are shown as means ± SD. P values are for differences between groups by Kruskal-Wallis test with Dunn's multiple comparison test. Abbreviations: EVLP = ex vivo lung perfusion; CIT = cold ischemic time.
Discussion
This study described the necessary steps for establishing a rat EVLP protocol. Here, we show that donor lungs can be perfused for 4 h after cold static preservation of up to 18 h at 4 °C. This was demonstrated by assessing lung compliance, pulmonary vascular resistance, perfusate glucose/lactate concentration, and P/F ratio.
The rise of the EVLP platform as an important tool in the evaluation of lung function and for graft reconditioning has motivated several groups to develop a rat EVLP model. These efforts are justified due to the lower cost and the possibility of biological manipulation, resulting in less sample variation, shorter experimental execution time, and wide possibilities for concept testing. Nevertheless, the establishment of reliable EVLP protocols in rats has proven to be tricky, with authors reporting difficulty in achieving stable perfusion and deterioration in lung function before 4 hours of EVLP9,11,13,14,15,16.
Addressed to these issues, researchers should be aware of possible air bubbles in the system that could favor episodes of pulmonary embolism. To do so, it needs to be ensured that the bubble trap is filled and that all connections are securely tied, preventing possible leaks. Besides this, during the development of the rat EVLP technique, the insertion of the PA and LA cannulas should be gentle, so that there is no torsion in the PA nor LA laceration, thus preventing interruption/invalidation of perfusion.
In addition to logistical factors, researchers must adhere to the perfusion protocol, as incorrect settings of the parameters can promote cyclical injuries turning perfusion unfeasible. The rat EVLP protocol described here is similar to clinical EVLP protocols, which utilize protective ventilatory and perfusion settings to avoid capillary compression by high alveolar pressure and prevent increases in pulmonary vascular resistance, as well as inflammatory markers7,13.
In conclusion, the rat EVLP model described here represents a potential tool in the study of lung grafts, allowing the evaluation in real time of the lung physiology and inflammatory changes associated with the time of perfusion, given the reproducibility and consistency of this protocol.
Disclosures
MC is a shareholder of Traferox Technologies Inc and consultant for Lung Bioengineering Inc. MC receives research support from Beyond Air Inc. and Synklino. The authors declare that there have been no competing interests that could have influenced the outcome reported in this paper.
Acknowledgements
Figure 1 was created with BioRender.com (confirmation of publication and licensing rights [Agreement: BT25KGSKWF]).
Materials
| Name | Company | Catalog Number | Comments |
| 14 G intravenous catheter | BD | 381167 | Orotracheal intubation |
| 16 G intravenous catheter | BD | 381157 | Catheter to flush the lung |
| 2-0 Suture Sofsilk | Covidien | S-305 | Tracheal tube fixation |
| 3-0 Suture Sofsilk | Covidien | S303 | Fixation of arterial and atrial cannulas |
| IPL-2 Core Isolated Perfused Lung System for Rat | Harvard Apparatus | 734276 | Ex Vivo Lung Perfusion (EVLP) system |
| Ketamine (Narketan) | Vetoquinol | 440894 | Sedation/anesthesia |
| Large Pulmonary Artery Cannula | Harvard Apparatus | 73-0711 | |
| Left Atrial Cannula | Harvard Apparatus | 73-0712 | |
| Low potassium dextran glucose solution (Perfadex) | XVIVO | 19811 | Preservation solution |
| Methylprednisolone sodium succinate (SOLU-MEDROL) | Pfizer | 14705 | Anti-inflammatory |
| Prostaglandin E1 (Prostin VR) | Pfizer | RX297945 | |
| Small Animal Ventilator (Model 683) | Harvard Apparatus | 55-0000 | |
| Sodium heparin | Leo Pharma | 453811 | |
| Steen Solution | XVIVO | 19004 | Buffered extracellular solution to perfuse lungs during EVLP |
| Sugita Aneur clip curv | Mizuho | 07-940-86 | Tracheal clamp |
| Tracheal Cannula | Harvard Apparatus | 73-3384 | |
| Xylazine (Rompun) | Bayer Healthcare | 2169592 | Sedation/anesthesia |
References
- Chambers, D. C., et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-eighth adult lung transplantation report — 2021; Focus on recipient characteristics. The Journal of Heart and Lung Transplantation. 40 (10), 1060-1072 (2021).
- Meyer, K. C. Recent advances in lung transplantation. F1000Research. 7, 1684 (2018).
- Valapour, M., et al. OPTN/SRTR 2018 Annual Data Report: Lung. American Journal of Transplantation. 20, 427-508 (2020).
- Chaney, J., Suzuki, Y., Cantu, E., van Berkel, V. Lung donor selection criteria. Journal of thoracic disease. 6 (8), 1032-1038 (2014).
- Watanabe, T., Cypel, M., Keshavjee, S. Ex vivo lung perfusion. Journal of Thoracic Disease. 13 (11), 6602-6617 (2021).
- Saddoughi, S. A., Cypel, M. Expanding the lung donor pool. Clinics in Chest Medicine. 44 (1), 77-83 (2023).
- Wang, A., Ali, A., Keshavjee, S., Liu, M., Cypel, M. Ex vivo lung perfusion for donor lung assessment and repair: a review of translational interspecies models. American Journal of Physiology-Lung Cellular and Molecular Physiology. 319 (6), L932-L940 (2020).
- Noda, K., Philips, B. J., Atale, N., Sanchez, P. G. Endothelial protection in lung grafts through heparanase inhibition during ex vivo lung perfusion in rats. The Journal of Heart and Lung Transplantation. 42 (6), 697-706 (2023).
- Nelson, K., et al. Method of isolated ex vivo lung perfusion in a rat model: lessons learned from developing a rat EVLP program. Journal of Visualized Experiments. (96), (2015).
- Malik, A. B., Kaplan, J. E., Saba, T. M. Reference sample method for cardiac output and regional blood flow determinations in the rat. Journal of Applied Physiology. 40 (3), 472-475 (1976).
- Noda, K., et al. Successful prolonged ex vivo lung perfusion for graft preservation in rats. European Journal of Cardio-Thoracic Surgery. 45 (3), e54-e60 (2014).
- Cypel, M., et al. Technique for prolonged normothermic ex vivo lung perfusion. The Journal of Heart and Lung Transplantation. 27 (12), 1319-1325 (2008).
- Ohsumi, A., et al. A method for translational rat ex vivo lung perfusion experimentation. American Journal of Physiology-Lung Cellular and Molecular Physiology. 319 (1), L61-L70 (2020).
- Soares, P. R. O., et al. Comparison between perfadex and locally manufactured low-potassium dextran solution for pulmonary preservation in an ex vivo isolated lung perfusion model. Transplantation Proceedings. 43 (1), 84-88 (2011).
- Pêgo-Fernandes, P. M., et al. Experimental model of isolated lung perfusion in rats: first Brazilian experience using the IL-2 isolated perfused rat or guinea pig lung system. Transplantation Proceedings. 42 (2), 444-447 (2010).
- Yamanashi, K., et al. Reduction of donor mononuclear phagocytes with clodronate-liposome during ex lung perfusion attenuates ischemia-reperfusion injury. The Journal of Thoracic and Cardiovascular Surgery. 165 (4), e181-e203 (2023).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved