Method Article
Utilizing Low Oxygen Tension to Reduce Hematopoietic Cells in Murine Bone Marrow Stromal Cell Cultures
In This Article
Summary
A routine culture of bone marrow stromal cells (BMSCs) leads to the isolation of heterogeneous cell populations, with many cells being of hematopoietic origins. Here, we describe a method that utilizes low oxygen tension to greatly reduce hematopoietic contaminants in murine BMSC cultures.
Abstract
Currently, there remains a lack of universally accepted markers to prospectively isolate a homogeneous population of skeletal stem cells (SSCs). For this reason, BMSCs, which support hematopoiesis and contribute to all the functions of the skeleton, continue to be widely used to study multipotent mesenchymal progenitors (MMPs) and to infer SSC function. Moreover, given the breadth of transgenic murine models used to study musculoskeletal diseases, the use of BMSCs also serves as a powerful tool to examine the molecular mechanisms regulating MMPs and SSCs. However, common isolation procedures for murine BMSCs result in over 50% of recovered cells being of hematopoietic origins, potentially hindering the interpretation of the data generated during these studies. Here, we describe a method using low oxygen tension or hypoxia for the selective elimination of CD45+ cells in BMSC cultures. Importantly, this method can be easily implemented to not only reduce hemopoietic contaminants but to also enhance the percentage of MMPs and putative SSCs in BMSC cultures.
Introduction
Similar to hematopoietic stem cells (HSCs), SSCs are housed within the bone microenvironment; however, unlike HSCs, currently there is a lack of universally accepted cell surface markers that can be used to prospectively identity SSCs1,2,3. However, in vitro culture systems and in vivo reconstitution assays demonstrate that a proportion of BMSCs have the capacity to support hematopoiesis, as well as the ability to differentiate into all cells of the mesenchymal lineages4,5. Thus, while BMSCs represent a highly heterogeneous population, a proportion of these cells have features of bona fide stem cells, as defined by their ability to self-renew and reconstitute all cells of their tissue of origin. Moreover, unlike the use of cell surface markers, BMSCs can be quickly isolated based on their rapid adherence to tissue culture plastic6,7. For these reasons, BMSCs are often used as a surrogate for SSCs5.
While preferential adherence to tissue culture (TC) plastic has been used to successfully isolate BMSCs from human tissue, murine models present additional challenges for this method. Most notably, as murine hematopoietic cells have the capacity to adhere to both TC plastic and to BMSCs, high hematopoietic contamination occurs in this model system, thus hindering the interpretation of the data generated using this isolation method6.
Notably, BMSCs reside in the bone microenvironment where oxygen tensions range from 1%-4%8. However, under standard tissue culture conditions, cell culture incubators are maintained at atmospheric oxygen levels of 21%, representing supraphysiological levels. Highlighting the functional significance of these differences, culturing cells of mesenchymal origins at 21% oxygen is associated with increased cell death9,10. Moreover, we have recently demonstrated that hematological cells and mesenchymal cells differentially respond to low oxygen tensions. Specifically, there is a substantial decrease in the numbers of CD45+ hematopoietic cells when BMSCs cultures are maintained at low oxygen tensions. Indeed, we noted a 90% reduction in CD45+ hematopoietic cells using this technique11.
Here, we share a protocol that can be easily implemented for labs to significantly reduce hematopoietic contamination and to improve the purity of mesenchymal cells during routine culture of BMSCs.
Protocol
All methods described utilizing murine models were performed in compliance with approved Institutional Animal Care and Use Committee (IACUC) protocols (A187-19-08).
1. Dissection of hindlimb bones
- Euthanize either 10-12-week-old male or female C57BL/6 mice by CO2 asphyxiation using a flow rate of 2-4 L/min followed by confirmation of death by decapitation. Either female or male mice can used in this assay with no expected differences in the results. Spray down fur with 70% ethanol.
- Use sterile surgical forceps and scissors to make a small incision in the skin underneath the sternum. Use hands to tear the skin around the circumference of the body and then pull the skin distally toward the hindlimbs to expose the underlying tissue.
- Use sterilized surgical forceps and scissors to gently remove the hindlimb by separating the femoral head (ball) from the acetabulum (socket) of the pelvis (hip). Use sterile forceps and a scalpel to gently scrape along the bones to remove as much soft tissue as possible.
- Use a sterile scalpel to separate the femur and tibia using minimal resistance to cut the ligaments (lateral and medial collateral, anterior and posterior cruciate, and patellar tendon [ligament]) connecting the two bones. Importantly, after separation, keep the condyles on both the distal femur and the proximal tibia intact.
- For distal femurs, make a cut between the femoral head and the third trochanter and a cut at the epiphysis proximal to the growth plate, minimizing damage to the growth plate.
- For proximal tibiae, make one cut proximal to the lateral malleolus and one cut distal of the tibial plateau just proximal to the growth plate.
- Place femurs and tibiae cut side down in individual 1.5 mL microcentrifuge tubes.
2. Isolation of BMSCs from femurs and tibiae
- Place 1.5 mL microcentrifuge tubes in a benchtop centrifuge and spin at 10,000 x g at 4 °C for 1 s.
- Use sterile tweezers to carefully remove bones from the microcentrifuge tubes, leaving a red plug of bone marrow at the bottom of the microcentrifuge tube. If some marrow remains in the bone shaft, transfer the bone to a new tube, repeat the quick spin, and combine the pellets in one tube during the resuspension Step 2.3.
- Use a P1000 pipette to gently resuspend the marrow pellet in 500 µL of growth media, MEMa supplemented with 20% fetal bovine serum and 1,000 U/ml penicillin-streptomycin. Transfer bone marrow stromal cells to a 15 mL conical tube containing 4.5 mL of growth media.
3. Lysis of red blood cells
- Spin down cells at 300 x g at 4 °C for 5 min. Aspirate the growth media and gently resuspend the pellet in 1.5 mL of red cell lysis buffer.
- Incubate at room temperature for 75-90 s. Immediately quench with 10 mL of growth media.
- Spin down cells at 300 x g at 4 °C for 5 min. Aspirate the media and resuspend the pellet in 10 mL of growth media for counting (see Step 4).
NOTE: The cell pellet should no longer be red but white, indicating successful lysis of red blood cells. If the pellet is still red, an additional round of red cell lysis buffer can be performed.
4. Plating BMSCs
- To count live cells for plating, remove 10 µL of cell suspension and add to 10 µL of Trypan Blue solution.
- Place 10 µL of cell:Trypan Blue solution mixture onto a hemocytometer and count the viable (non-blue) cells. Most cells should exclude Trypan blue.
- For colony assays, plate the cells at a density of 60,000 cells/cm2 into T-25 cm2 tissue culture flasks. For high density BMSC cultures, plate cells at a density of 130,000 cells/cm2 into 100 mm2 tissue culture dishes. Use a total volume of 5 mL for T-25 cm2 flasks and a total volume of 10 mL for 100 cm2 dishes.
- Incubate the cells for 3 h at either 1%-2 % (hypoxia) or 21% oxygen (normoxia) and 5% CO2. For routine culture of BMSCs at low oxygen tensions, either a hypoxic cell incubator or a hypoxia chamber can be used. Set each instrument according to the manufacturer's instructions so that the gas flow of nitrogen and carbon dioxide will result in the desired range of oxygen and CO2.
- After 3 h, carefully rinse the plates 3x with 1x PBS. To avoid disrupting cells that have begun to attach, gently rinse by slowing aspirating and pipetting onto the side of the tissue culture dishes.
- Following the final 1x PBS rinse, replace the PBS with 10 mL of growth media if using 100 cm2 dishes or 5 mL of growth media if using T-25cm2 flasks. After rinsing, most floating cells should be removed.
5. Maintaining BMSCs in hypoxic conditions
- For hypoxic cultures, maintain cells in a hypoxia chamber or incubator set to 1%-2% O2 and 5% CO2. For normoxic cultures, maintain cells at 21% O2 and 5% CO2. If using a hypoxic incubator, ensure that the doors are opened minimally to prevent influxes of atmospheric oxygen (21%). Change the media every 2-3 days.
- For colony assays, incubate the cells for 7-10 days, after which either enumerate the colonies or begin differentiation or other desired assays. Under hypoxic conditions, between 50-120 colonies can be anticipated, whereas under normoxic conditions, between 5-30 colonies can be anticipated.
- For high-density BMSC cultures, incubate the cells until confluent, approximately 7-10 days for hypoxic cultures and approximately 14-21 days for normoxic cultures. Once confluent, trypsinize the cells with 0.25% trypsin for use in the desired assays.
- To visualize the cells, place the tissue culture dish on a stage, rotate the phase objective into the optical pathway, and ensure the phase contrast slider is selected for the corresponding objective. View the cells at either 100x or 200x magnification.
Results
After 7 days of cell isolation, cells cultured at 21% oxygen are highly heterogeneous. Specifically, there is a large variation in size, with larger bipolar cells interspaced with smaller cells containing multiple protrusions (Figure 1A). In contrast, cells grown in hypoxic conditions are highly homogeneous. Cells within colonies are relatively similar in size and have a bipolar appearance, resembling other cells of mesenchymal origins grown on tissue culture plastic (Figure 1B). At clonogenic density 14 days post culture, cells maintained in normoxic cultures have approximately 5-30 colonies per T25cm2 flask, which are smaller in size when compared to colonies grown in hypoxic conditions (Figure 2A). Notably, cells grown in hypoxia proliferate as marked by robust colony formation, which ranges from 50-120 colonies per T25cm2 flask (Figure 2B). Moreover, at 21% oxygen, cultured cells display the hallmark morphological features of senescent cells, appearing large, flat, and multinucleated (Figure 3). Additionally, β-galactosidase staining can be performed to definitively identify senescent cells in culture12.
Confirmation of cell types can be accomplished using flow cytometry for the presence of the hematopoietic cell surface marker, CD45+. Under normoxic conditions, greater than 50% of cells express the cell surface marker CD45+, while under hypoxic conditions, 5%-10% of cells express this marker, as shown in Figure 4 and in a previous study11. Moreover, confirmation of MMPs can be performed by examining the expression of markers such as PDGFRα, SCA-1, CD73, CD90, and CD146 by flow cytometry11,13,14,15.

Figure 1: BMSC cultured in 1% or 21% oxygen. Representative phase contrast images of BMSC isolated from 12-week-old C57BL/6 male mice and cultured in either (A) 21% oxygen (normoxia) or (B) 1% oxygen hypoxia for 7 days. White arrows denote small hematopoietic cells. Please click here to view a larger version of this figure.
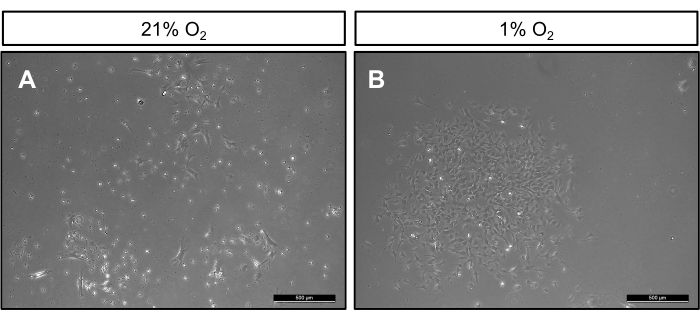
Figure 2: BMSCs plated at clonogenic density in 1% and 21% oxygen. Representative phase contrast images of BMSCs isolated from 12-week-old C57BL/6 male mice plated at a clonogenic density of 60,000 cells/cm2. Cells grown in either (A) 21% oxygen or (B) 1% oxygen. Please click here to view a larger version of this figure.
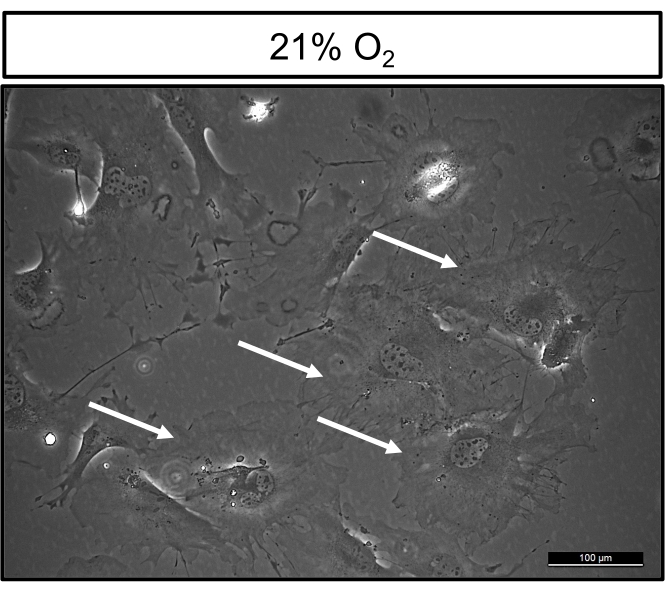
Figure 3: Cells cultured in 21% oxygen display morphological features of senescent cells. Phase contrast image of BMSCs isolated from 12-week-old C57BL/6 male mice and grown at 21% oxygen. White arrows denote cells that are large, flat, and multinuclear, which are the morphological characteristics of senescent cells. Please click here to view a larger version of this figure.

Figure 4: Flow cytometry analysis of CD45+ cells in BMSC cultures. Representative flow cytometry plots of BMSCs isolated from 12-week-old C57BL/6 male mice grown in either 21% O2 or 1% O2 and stained for CD45+. Please click here to view a larger version of this figure.
Discussion
Based on our observations, BMSCs adhere to tissue culture plastic earlier than macrophages and other cells of hemopoietic origins11. For this reason, rinsing plates 3 h post plating is a critical step as this removes floating hematopoietic cells that have the potential to attach later time points. Moreover, these rinses should be done with care, specifically by pipetting media onto the side of the dish to prevent the disruption of loosely attached, rounded BMSCs that have not yet laid down matrix and spread on the tissue culture dishes. After three rinses, most floating cells should be removed. While rinsing BMSC cultures at this time point diminishes CD45+ cells, maintaining cultures in hypoxia is required to both significantly decrease the percentage of hematopoietic cells and to increase the percentage of stromal cells11. Importantly, if colonies fail to appear after 72 h, it is likely the rinses were too harsh and both BMSCs and hematopoietic cells were removed.
Hypoxic cultures should appear morphologically homogenous by day 7 (Figure 1 and Figure 2), containing large, bipolar, fibroblastic cells. Importantly, this method does not isolate SSCs or a functionally homogenous population of MMPs. Rather, this method increases the proportion of BMSCs expressing the known SSC cell surface marker, PDGFRα, and decreases the percentage of CD45+ cells in cultures11. Importantly, subculturing, immunodepleting, and seeding cells at low density have also been employed to purify MMPs; however, these methods can result in senescent phenotypes, altered differentiation, and the selection of clones, leading to cellular transformation9,16,17. While this technique does decrease the number of hematopoietic contaminants, one limitation is that it does not isolate a heterogeneous population of cells, nor does it eliminate CD45+ hematopoietic cells. Thus, while further purification using cell surface markers expressed by SSCs can be used, the use of low oxygen tensions for the purification of stromal cell populations is rapid, reliable, and easily implemented.
Oxygen tension in the bone microenvironment ranges from 1%-4% 8. We observed that oxygen tensions of either 2% or 1% decrease the number of CD45+-expressing cells present in BMSC cultures. However, it should be noted for any subsequent experiments, such as differentiation assays, that the oxygen tension will need to be determined by the individual researcher. It is important to note that the differentiation potential of BMSCs can vary depending on oxygen levels18. This may be due in part to the sensitivity of the HIF signaling pathway, which influences BMSC differentiation, to different oxygen tensions19. Hence, by purifying for MMPs this method should improve the interpretation of results for experiments utilizing BMSCs.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Department of Orthopaedic Surgery at Duke University.
Materials
| Name | Company | Catalog Number | Comments |
| 100mm2 tissue culture dishes | Corning | 353003 | |
| 1x Phosphate Buffred Saline (PBS) | Gibco | 100010-023 | |
| Bright-Line hemocytometer | Sigma-Aldrich | Z359629 | |
| C57BL/6J | The Jackson Laboratory | 664 | |
| HyClone Fetal Bovine Serum (U.S.), Characterized | GE Healthcare Life Sciences | SH30071.03 | |
| InvivO2 400 | Baker Ruskinn | https://bakerco.com | |
| MEMα, nucleosides | Gibco | 12571-063 | |
| Penicillin-Streptomycin (10,000 U/mL) | Gibco | 15140-122 | |
| Red Blood Cell Lysing Buffer Hybri-Max | Sigma-Aldrich | R7757 | |
| T-25cm2 tissue culture flasks | Corning | 430168 | |
| Trypan Blue Solution | Sigma-Aldrich | T8154 | |
| Trypsin-EDTA (0.25%), phenol red | Gibco | 25200-056 |
References
- Chen, K. G., Johnson, K. R., Robey, P. G. Mouse genetic analysis of bone marrow stem cell niches: technological pitfalls, challenges, and translational considerations. Stem Cell Reports. 9 (5), 1343-1358 (2017).
- Baryawno, N., et al. A cellular taxonomy of the bone marrow stroma in homeostasis and leukemia. Cell. 177 (7), 1915-1932 (2019).
- Tikhonova, A. N., et al. The bone marrow microenvironment at single-cell resolution. Nature. 569 (7755), 222-228 (2019).
- Friedenstein, A. J., Chailakhjan, R. K., Lalykina, K. S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinetics. 3 (4), 393-403 (1970).
- Bianco, P., Riminucci, M., Gronthos, S., Robey, P. G. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 19 (3), 180-192 (2001).
- Bearpark, A. D., Gordon, M. Y. Adhesive properties distinguish sub-populations of haemopoietic stem cells with different spleen colony-forming and marrow repopulating capacities. Bone Marrow Transplantation. 4 (6), 625-628 (1989).
- Kerk, D. K., Henry, E. A., Eaves, A. C., Eaves, C. J. Two classes of primitive pluripotent hemopoietic progenitor cells: separation by adherence. Journal Cell Physiology. 125 (1), 127-134 (1985).
- Spencer, J. A., et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 508 (7495), 269-273 (2014).
- Boregowda, S. V., et al. Atmospheric oxygen inhibits growth and differentiation of marrow-derived mouse mesenchymal stem cells via a p53-dependent mechanism: implications for long-term culture expansion. Stem Cells. 30 (5), 975-987 (2012).
- Parrinello, S., et al. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nature Cell Biology. 5 (8), 741-747 (2003).
- Guo, W., et al. Hypoxia depletes contaminating CD45(+) hematopoietic cells from murine bone marrow stromal cell (BMSC) cultures: Methods for BMSC culture purification. Stem Cell Research. 53, 102317 (2021).
- Dimri, G. P., et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. The Proceedings of the National Academy of Sciences of the United States of America. 92 (20), 9363-9367 (1995).
- Naserian, S., Shamdani, S., Arouche, N., Uzan, G. Regulatory T cell induction by mesenchymal stem cells depends on the expression of TNFR2 by T cells. Stem Cell Research and Therapy. 11 (1), 534 (2020).
- Razazian, M., et al. Differences and similarities between mesenchymal stem cell and endothelial progenitor cell immunoregulatory properties against T cells. World Journal of Stem Cells. 13 (8), 971-984 (2021).
- Ambrosi, T. H., Longaker, M. T., Chan, C. K. F. A revised perspective of skeletal stem cell biology. Frontiers in Cell and Developmental Biology. 7, 189 (2019).
- Krebsbach, P. H., Kuznetsov, S. A., Bianco, P., Robey, P. G. Bone marrow stromal cells: characterization and clinical application. Critical Reviews in Oral Biology and Medicine: An Official Publication of the American Association of Oral Biologists. 10 (2), 165-181 (1999).
- Harvey, D. M., Levine, A. J. p53 alteration is a common event in the spontaneous immortalization of primary BALB/c murine embryo fibroblasts. Genes and Development. 5 (12), 2375-2385 (1991).
- Buravkova, L. B., Andreeva, E. R., Gogvadze, V., Zhivotovsky, B. Mesenchymal stem cells and hypoxia: where are we. Mitochondrion. 19, 105-112 (2014).
- Kaelin, W. G., Ratcliffe, P. J. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Molecular Cell. 30 (4), 393-402 (2008).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved