Method Article
Real-Time Magnetic Resonance Guided Focused Ultrasound for Painful Bone Metastases
In This Article
Summary
Magnetic resonance could offer real-time monitoring of the position and temperature of focused ultrasound in thermal ablation for painful bone metastases, regardless of cancer type or previous local treatments. Our innovative method of quality assurance could facilitate the application of this effective and safe treatment.
Abstract
Bones are one of the most common sites of cancer metastasis, which usually causes pain and impairs quality of life. Radiation therapy combined with opioids is the standard treatment for painful bone metastases. This treatment achieves effective pain control in 60−74% of patients, but limited treatment choices with limited benefits are available for recurrent or residual painful bone metastases after radiotherapy. More than 40% of patients still experience moderate to severe bone pain after reirradiation. Magnetic resonance-guided focused ultrasound (MRgFUS) combines high-intensity focused ultrasound, which achieves thermal ablation of bone metastases and subsequent pain reduction, with real-time magnetic resonance (MR) thermometry to monitor the temperature of anatomic MR images, with an accuracy of 1 °C, spatial resolution of 1 mm, and temporal resolution within 3 s. As well as being increasingly used clinically for controlling metastatic bone pain, the use of MRgFUS for other diseases has also been tested. However, the use of MR software as a thermometer is the only technique available to verify the accuracy of the software and assure energy delivery. Here, we describe an efficient method of quality assurance we developed for thermal detection and energy delivery before each MRgFUS treatment and also propose a modified workflow to expedite the treatment course as well as to reduce patients' pain during the procedure.
Introduction
Bones are one of the most common sites of cancer metastasis, which usually causes pain and impairs quality of life. Radiation therapy (RT) combined with opioids is the standard treatment for painful bone metastases. This treatment achieves effective pain control in 60−74% of patients1. However, limited treatment choices are available for recurrent or residual metastatic bone pain after RT. Reirradiation, surgical intervention, percutaneous cryoablation, or radiofrequency ablation and increased doses of systemic opioids and analgesics are options with limited indications and usually with side effects. Moreover, these secondary treatments have yielded unsatisfactory results: more than 40% of patients continue to experience moderate to severe bone pain after reirradiation2.
High-intensity focused ultrasound systems integrate ultrasounds from multiple angles into one spot, transferring acoustic energy at ablative temperatures of more than 65 °C3. This noninvasive technique has been used for thermal ablation at various sites and for various types of lesions4,5. Generally, focused ultrasound systems generate acoustic energy at frequencies of 200 kHz-4 MHz6,7, producing an intensity in the focal point on the order of 100-10,000 W/cm2. At these energy levels, the focused ultrasound beams trigger a rise in cell temperature over the treated volume of tissue. The temperature rise varies according to the tissue absorption coefficient, predicted using Arrhenius analysis or the Sapareto-Dewey isoeffect thermal dose relationship. To achieve better control and a more rapid temperature increase, focal volumes of 0.2−5 mm3 are suggested for each sonication. Therefore, the ablation of larger areas requires tiling of multiple sonications to cover a large volume and to create homogeneous thermal damage. In addition to causing damage as a result of thermal effects, focused ultrasound also creates microbubbles because of physical factors such as rectified diffusion in the treated area. When the size of microbubbles reaches a cutoff, they eventually implode, causing microshock waves and affecting surrounding tissues. This parallel nonthermal effect also contributes to tissue injury and tumor necrosis.
Unlike other image guidance techniques, such as ultrasound imaging, magnetic resonance (MR) imaging provides a three-dimensional image of anatomy with clear resolution images of soft tissue and quantitative temperature monitoring. The mapping software of quantitative MR thermometry can calculate the thermal change in degrees Celsius and then superimpose the respective locations onto the anatomic MR images8. By detecting the proton resonance frequency shift in water hydrogen, which corresponds to approximately 0.01 ppm per degree Celsius, the temperature-sensitive MR sequence can control energy deposition, with an accuracy of 1 °C for measurement of thermal changes, a spatial resolution of 1 mm, and a temporal resolution within 3 s9,10. With this extended software, the MR device could provide diagnostic images and also detect thermal changes within seconds, mapping these onto the anatomical images during the whole treatment course. Despite the development of such an innovative technique, few articles describe qualitative security during each treatment course. Here we aim to share our protocol and experiences with MRgFUS.
Protocol
Taipei Medical University Joint Institutional Review Board approval was obtained for this study.
NOTE: The same protocol, validated in Kao et al.11, has been used to treat 138 cases between 2015 and 2019. The inclusion criteria for treatment enrollment were 1) the presence of a solitary distinguishable painful bone metastasis; 2) no administration of previous local therapy to the targeted bone lesion; and 3) the ability to access the targeted bone lesion with MRgFUS (Table of Materials). Patients with impending pathological fractures were excluded. Detailed materials and devices are listed in the Table of Materials.
1. Pretreatment consultation and CT-simulation for treatment spot
- Evaluation of patients indicated for MRgFUS
- Assess patient suitability for MRgFUS in treating metastatic bone pain. Explain the procedure and related information to the patient and family. Record daily analgesic medication and pain score before and after medication.
- Have a radiation oncologist and radiologist locate the lesion and nearby anatomy based on pretreatment computed tomography (CT) or magnetic resonance imaging (MRI) scans.
NOTE: Spinal metastasis is excluded because of possible injury to the spinal cord or cauda equina. Lesions in the trunk should be treated dorsally rather than ventrally in order to prevent injury to major vessels and organs.
- Confirm the treatment spot by CT simulation 1 day before MRgFUS.
- Position the patient in a supine, head-first position on the couch and perform a helical CT scan (120 kV, 400 mAs/slice) over the treating area with a 3 mm slice thickness. Adjust and tilt the patient's position, in the center of the couch, to locate the lesion.
- Place a CT marker, 1 cm lead wire, on the skin surface, vertically closest to the lesion, and conduct a helical CT scan (120 kV, 400 mAs/slice) again to confirm the position of the patient and the location of the CT marker. Mark the location of the CT marker with a marker pen and take a picture of the patient's position.
NOTE: Precise confirmation of the treatment spot and position before MRgFUS can facilitate the positioning process during MRgFUS.
2. Patient preparation for MRgFUS on treatment day
- Verify the patient's identity according to photo identification. Verify that the patient removed all metal objects and magnetic devices prior to the scan.
- Prescribe local and systemic analgesics before treatment.
- At 1 h before the scheduled treatment time, apply lidocaine cream on the marked skin, with a radius of 10 cm. Remove the cream carefully 10 min before treatment.
- At 30 min before treatment, intravenously drip 5 mg of dexamethasone with 50 mL of normal saline for 10 min and 30 mg of ketorolac with 50 mL of normal saline for 10 min. Set the peripheral intravenous line on either hand, forearm, leg, or foot on the side opposite to the lesion.
- Check the patient's vital signs (heart rate, blood pressure, respiratory rate, and blood saturation) 5 min before sending the patient out for treatment.
3. Daily quality assurance (DQA) before MRgFUS
- DQA setup
- Replace the diagnostic couch with the MRgFUS couch with a focused ultrasound transducer and connect the couch to the system.
NOTE: The staff must remove all metal objects and electric devices, including rings, watches, pens, mobile phones, or magnetic ID cards, before entering the MRI room. - Apply ultrasound transmission gel (~1 mm thickness) and degassed water on the surface panel of the focused ultrasound transducer.
NOTE: Be careful not to scratch the plastic panel during this process. - Carefully cover the panel with plastic drape, avoiding any folds of the drape over the panel area. Add degassed water to a level as high as the MR coil on the couch.
NOTE: Be careful not to make any gas bubbles between the panel, transmission gel, the drape, and degassed water. - Slowly and carefully place the gel pad on the panel without creating any gas bubbles during the process. Place the DQA phantom on the gel pad without creating any gas bubbles.
- Place the MR coil on the couch and connect the coil to the MRI device. Press Landmark on the MRI control panel and align the red laser to the black stripe on the coil. Then, press Advanced to Scan on the MRI control panel.
- Replace the diagnostic couch with the MRgFUS couch with a focused ultrasound transducer and connect the couch to the system.
- DQA prescan
- Click Idle on the MRI system to create a new MR scan. Enter DQA as patient's name and enter 50 kg for body weight. Choose Supine and Feet First as scanning parameters.
- Choose scanning protocol as ExAblate - Plan - Bone. Then click Save Series | Download | Scan. Check scanning images on the monitor to check for any gas bubbles.
NOTE: If any gas bubbles are found, set up the DQA once more to remove them.
- DQA procedure
- Click Bone Tumors on the MRgFUS system and click Calibrate to start the DQA. Click MR Scan and confirm that the exam number is the same as in the MRI system.
- Adjust the position of the transducer in the axial and sagittal images in order to let the sonication field cover the phantom. Click Load to load MRI images. Then click Sag | Select All to select all images. Click Ax | Select All again.
- Click Draw to define the sonication area. Click Skin Line to contour the surface between the phantom and gel pad. Click Copy to copy the skin lines to all sagittal and axial slices of images. Then adjust and confirm the skin line is correct in each image.
- Click Treating Area to contour the treatment area in the phantom for three continuous slices. Click Protocol to choose Bone 15 and then click Apply. Click Fiducial and choose a spot in the phantom as a reference point.
- DQA planning
- Click Plan | Verify to proceed. Click Add Sonication to add one spot for sonication within the phantom. Confirm that the sonication field is within the phantom in each sagittal and axial slice.
- Set the scan parameters: direction = Coronal and number slice = 5 with preset energy output. Click Sonication to start.
- Calibration
- After sonication, monitoring the MRgFUS system shows the temperature images. Confirm the heating spot and click Center to mark the spot. Use the mouse to check the heating spot and other, different spots to compare the thermal curve to locate artifacts or background signals.
- The system shows adjustments for transducer location in millimeters in 3 axials. Click Accept and then click Back to perform the sonication again with a 20% increase in energy. Confirm that the 2nd adjustment is within 1 mm and click Reject.
- Set scan parameter: direction = Axial and number slice = 5 with preset energy output. Make axial adjustments as described in steps 3.5.1 and 3.5.2. Click Exit to leave DQA and remove the phantom.
4. Patient positioning and pretreatment MR scanning
- Patient positioning
- Position the patient on the MRgFUS couch in the same position as the previous simulation in step 1.2. Align the mark on the skin with the center of the gel pad.
- Secure the patient to the couch using a safety belt and teach the patient how to use the emergency button. Set the finger pulse oximeter on one index finger.
- Place the MR coil on the couch and align the coil. Then press Advanced to Scan on the MRI control panel.
- Pretreatment MR scanning
- Create a new MR scan and enter the patient's information. Choose Supine and Feet First as the scanning parameters and the scanning protocol as ExAblate - Plan - Bone.
- Acquire three-plane T2 images, then click View Edit to confirm the scanning area. Click Save | Download | Auto PreScan. Confirm the scanning area after prescan and then click Scan.
- Confirm the lesion and patient position.
- Reconfirm the lesion, MR scanning field, and patient position.
NOTE: The MR scanning field should be over the treatment area and cover the ultrasound transducer. - Examine any gas bubbles between skin surface and gel pad. Reposition the patient if any gas bubbles are present.
- Reconfirm the lesion, MR scanning field, and patient position.
5. Treatment contouring and planning
- Import MR images
- Click Bone Tumors on the MRgFUS system. Click Contouring | MR scan and confirm that the exam number is the same as in the MRI system.
- Click Load to load MRI images collected in step 4.2. Click Sag | Select All. Then click Ax | Select All again.
- Contouring
- Click Draw to define the sonication area. Click Skin Line to contour the skin surface. Click Copy to copy the skin lines to all sagittal and axial slices of the images. Adjust and confirm that the skin line is correct in each image.
NOTE: The skin line must be contoured on each MR image slice with the ultrasound transducer. - Click Bone to contour the bone surface. Click Block to contour vital organs, such as nerves, vessels, or bowels, to prevent sonication through these areas. Click Fiducial and choose a spot near the lesion as a reference point.
- Click Draw to define the sonication area. Click Skin Line to contour the skin surface. Click Copy to copy the skin lines to all sagittal and axial slices of the images. Adjust and confirm that the skin line is correct in each image.
- Planning
- Click Plan | Verify to proceed after all contouring is completed. Review treatment planning and adjust the sonication if needed.
NOTE: The pathway of sonication should be from the transducer to the lesion through the skin surface.
- Click Plan | Verify to proceed after all contouring is completed. Review treatment planning and adjust the sonication if needed.
6. Verification and treatment
- Analgesics and sedation
- At 10 min before verification and treatment, intravenously drip 25 mg of meperidine and 7.5 mg of midazolam with 50 mL of normal saline for 10 min.
- Intravenously drip 7.5 mg of morphine with 50 mL of normal saline for 10 min at an interval of 30 min if the patient complains of pain during the treatment course.
NOTE: A physician may adjust analgesics and sedation medication according to clinical conditions. - Periodically check the pulse and oximeter between sonication.
NOTE: If the patient is very nervous or requires accompaniment, a nurse or staff member may stay inside during sonication. MR and ultrasound do not cause radiation nor harm to other personnel nearby.
- Verification
- Choose one sonication with preset parameters and click Sonication to start. Monitor the temperature rise and thermal curve of the heating spot as well as the reference spot to check for artifacts or background signals. Increase the energy output and repeat the sonication to the same spot.
- Repeat the sonication to the same spot until the temperature is over 65 °C to reach thermal ablation.
NOTE: Different people with different body mass, different locations, and different tissues would have various energy absorptions and thermal changes. Using lower energy for verification is necessary.
NOTE: Repeatedly heating the same spot or a nearby area in a short time may influence MR thermometry. Therefore, allow the system to pause if the sonication intervals are too close.
- Treatment
- Click Sonication to start the treatment with the verified energy output described in step 6.2.
- Monitor the temperature rise and thermal curve of the heating spot and repeat the sonication with increasing energy output until the temperature is over 65 °C. Complete all sonication for the treatment area.
7. Post-treatment evaluation
- Post-treatment MR scanning
- Conduct post-MRgFUS scanning with all series as in steps 4.2 and 4.3.
- Inject intravenous contrast medium at a rate of 4−5 mL/s and conduct a contrasted MR scanning as step 7.1.1.
NOTE: The volume of contrast is based on body weight (i.e., 0.2 mL per 1 kg).
- Evaluate treatment/thermal effect from post-treatment MRI. Repeat the sonication if the thermal ablation does not treat the whole lesion.
Results
A 68-year-old male patient was diagnosed with hepatocellular carcinoma (HCC) in October 2012. He received a left lobectomy on October 18, 2012, and pathology reported an 8.8 cm HCC. After operation, he experienced lower back pain and soreness, and an MRI on November 2, 2012 revealed a large metastatic mass involving the left sacrum, ilium, and gluteal soft tissue. Because of tumor compression and pain reaching 6 points on the visual analogue scale (VAS), he received RT with 45 Gy in 15 fractions in November 2012, and systemic therapy for metastatic HCC was also prescribed. Six months later, the pelvic metastatic tumor progressed and pain recurred, reaching 7 points on the VAS. A second RT with 25 Gy in 10 fractions in June 2013 and a third RT with 25 Gy in 10 fractions in November 2013 were arranged to treat the progressing tumor. The pain subsided for another 4 months but then recurred, reaching 7 points on the VAS in May 2014.
Because irradiation had previously been administered three times in the same location, MRgFUS was the only treatment option. For a huge pelvic mass over the left side of the pelvis, the treatment on May 27, 2014 used nine sonications of 2987.56 ± 1083.98 J, heating the tumor up to 61.78 ± 7.11 °C in each 20 s sonication (Figure 1). Using CTCAE version 4.0, a Grade 1 skin burn with minimal symptoms was noted, but no intervention was required. The patient's pain level dropped to 4 points on the VAS, which allowed analgesic dosages to be reduced for over 3 months.
However, due to the failure of systemic medication, the residual mass progressed again and caused moderate to severe pain, intermittently reaching 8 points on the VAS 5 months after his first MRgFUS treatment. In the absence of alternatives, the second MRgFUS treatment (Figure 2) was arranged on January 11, 2015 for the same bone metastasis. The treatment plan used 5 sonications with 1638.60 ± 210.67 J, heating the tumor to 64.40 ± 6.31 °C in each 20 s sonication. No adverse effect was noted on this occasion. The patient's pain level decreased to 4 points on the VAS within 1 day, and he was continuously maintained at a level of <4 points on the VAS for over 3 months. He passed away 7 months after the second MRgFUS.

Figure 1: MR image in the 1st treatment. (A) Upper left image shows T2 fat-saturation before treatment and upper right image (B) shows T1 with contrast. The red arrowhead indicates the metastatic tumor over left sacroiliac joint. The lower image (C) is the monitoring image during the treatment, with the left side showing the current sonication spot and the right side showing the energy output and temperature of the sonication spot. Please click here to view a larger version of this figure.
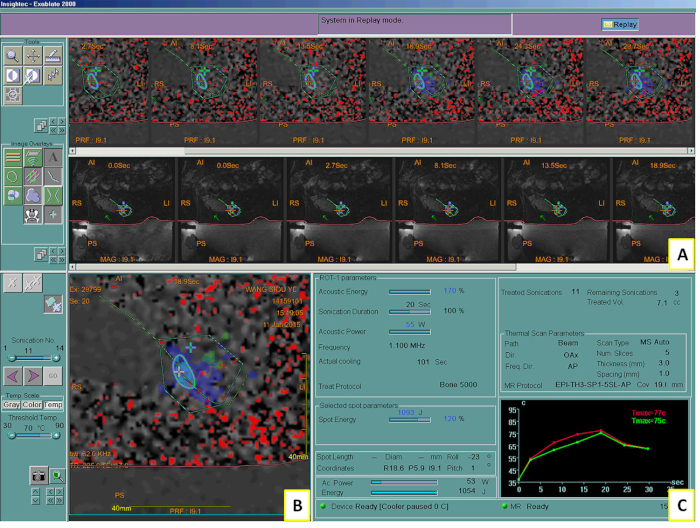
Figure 2: MRgFUS system showing in the 2nd treatment. System screen showing the intraprocedural MR images and controls (A), the thermal map after sonication (B), and a graph of the calculated temperature elevation during the sonication (C). Tmax = maximum temperature. Please click here to view a larger version of this figure.
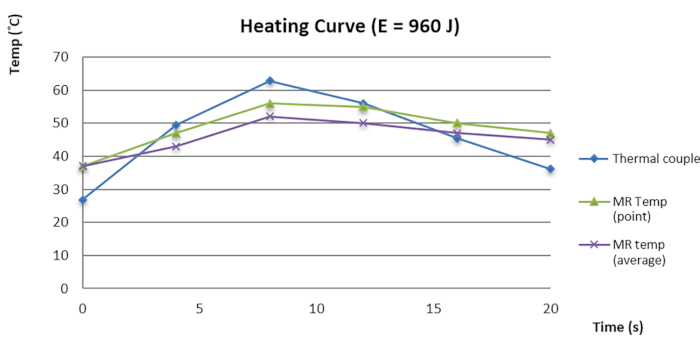
Figure 3: Temperature-time curve of MR thermometry and thermoelectric couple. Please click here to view a larger version of this figure.
Discussion
Several studies have demonstrated that MRgFUS is safe and efficient for controlling pain from recurrent or residual bone metastases after RT12,13. For 64.3-72.0% of patients, metastatic bone pain persists after RT and opioids. Studies have also determined MRgFUS has limited toxicity and a tolerable treatment course.
MRgFUS received approval for use in metastatic bone pain in 2011 by the Conformité Européenne and in 2012 by the U.S. Food and Drug Administration. As well as being increasingly used clinically for controlling metastatic bone pain, MRgFUS has also been investigated for use in other diseases, such as prostate cancer, breast cancer, and essential tremor9. However, use of MR software as a thermometer is the only technique available to verify the accuracy of the software and the safety of the device, which generates a focused ultrasound and delivers energy. Therefore, we demonstrated a treatment course using MRgFUS to treat bone metastases and also investigated an efficient method of providing quality assurance for thermal detection and energy delivery prior to each treatment. In this article, we propose modifications to the workflow currently recommended, which with the help of computed tomography simulation before treatment, could expedite the treatment course and also reduce patient suffering and pain during the procedure.
In our internal investigation, we found the focus error (FE) between the sonication focus and the spot with the highest temperature in the phantom was 1.73 ± 1.21 mm in the right-left (RL) axial, 0.95 ± 0.82 mm in the superior-inferior (SI) axial, and 0.31 ± 0.63 in the anterior-posterior (AP) axes before data quality assurance (DQA). After DQA, FE was significantly reduced to 0.43 ± 0.34 in the RL axial and 0.11 ± 0.22 in the SI axial, with p < 0.01 (pair t test). Our investigation suggested that DQA improves FE by up to 1 mm, with a 95% confidence interval, resulting in an FE of less than 0.5 mm in the SI and AP axes. Furthermore, we also verified the MR thermometry of MRgFUS with an MR-compatible thermoelectric couple (TEC) within a phantom to detect thermal changes. The result suggested that the thermal curve and temperature detection followed the same trend (Figure 3). The small temperature difference between MR thermometry and TEC contributed to quality assurance. Because the TEC is small and the metallic component interferes with image resolution, contouring the exact position of the TEC was difficult. Additional modification of the TEC in the phantom to improve thermal mapping and detection requires further investigation.
In conclusion, MRgFUS appears to be an effective, instant, and safe palliative treatment in patients with metastatic bone pain, especially for recurrent or residual pain. Demand and use for the treatment have been rapidly growing, but quality assurance and improvements to the treatment workflow have been rarely discussed in studies. Here, we describe our procedure and study results for DQA, indicating the value of DQA before each treatment. Using CT simulation before MRgFUS could facilitate workflow and reduce patients' suffering and pain during the procedure.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors thank Renyi Wang, medical physicist, for her help with investigating DQA.
Materials
| Name | Company | Catalog Number | Comments |
| 1L degasseed water pouch | InSightec | ASM001480 | for good ultrasound beam transmission |
| CT scan | Philips | Brilliance Big Bore 16 Slice CT, 7387 | Acquire CT images for positioning |
| EXABLATE | InSightec | EXABLATE 2000 | System for non-invasive tumor ablation through Focal Ultrasound (FUS) treatment under Magnetic Resonance (MR) guidance |
| Gel Pad ASSY | InSightec | SET999014 | Transmission gel pad for single Body treatment. |
| MR scan | GE | HDxT | Acquire MR images for contouring and planning |
| MRI contrast | Guerbet | Dotarem | Enhance MR for acquiring images |
| Patient accessory kit | InSightec | SET000016 | clinical applications single use treatment kit |
| Patient plastic drape | InSightec | DTP000067 | Cover the panel of ultrasound transducer. Deposible, hygiene use |
| Pelvic RF coil | GE | ASM000956 | Enhance MR for acquiring images |
| phantom | ATS Labs ATS Labs Inc | Model TxS-100 | for calibration |
| ultrasound transmission gel | InSightec | SET000885 | gel for calibration prior MR-guided FUS treatment |
References
- Lutz, S., et al. Palliative radiation therapy for bone metastases: Update of an ASTRO Evidence-Based Guideline. Practical Radiation Oncology. 7 (1), 4-12 (2017).
- Selvaggi, G., Scagliotti, G. V. Management of bone metastases in cancer: a review. Critical Reviews in Oncology/Hematology. 56 (3), 365-378 (2005).
- Simon, C. J., Dupuy, D. E., Mayo-Smith, W. W. Microwave ablation: principles and applications. Radiographics. 25 (Suppl 1), S69-S83 (2005).
- Napoli, A., et al. MR imaging-guided focused ultrasound for treatment of bone metastasis. Radiographics. 33 (6), 1555-1568 (2013).
- Jolesz, F. A., Hynynen, K. Magnetic resonance image-guided focused ultrasound surgery. Cancer Journal. 8, S100-S112 (2002).
- Umemura, S., Kawabata, K., Hashiba, K. Enhancement of ultrasonic absorption by microbubbles for therapeutic application. , (2001).
- Tran, B. C., et al. Microbubble-enhanced cavitation for noninvasive ultrasound surgery. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 50 (10), 1296-1304 (2003).
- Rieke, V., Butts Pauly, K. MR thermometry. Journal of Magnetic Resonance Imaging. 27 (2), 376-390 (2008).
- Poorter, J. D., et al. Noninvasive MRI thermometry with the proton resonance frequency (PRF) method: in vivo results in human muscle. Magnetic Resonance in Medicine. 33 (1), 74-81 (1995).
- Gorny, K. R., et al. MR guided focused ultrasound: technical acceptance measures for a clinical system. Physics in Medicine & Biology. 51 (12), 3155 (2006).
- Kao, Y. T., et al. Position stability analysis of a clinical mri-guided focused ultrasound system: one-year experience. Therapeutic Radiology and Oncology (In Traditional Chinese). 23 (2), 107-114 (2016).
- Bazzocchi, A., et al. MRI-guided focused ultrasound surgery in musculoskeletal diseases: the hot topics. The British Journal of Radiology. 89 (1057), 20150358 (2016).
- Gennaro, N., et al. Thermal ablation to relieve pain from metastatic bone disease: a systematic review. Skeletal Radiology. 48 (8), 1161-1169 (2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved