Method Article
Murine Model of Thoracic Aortic Dissection Induced by Oral β-Aminopropionitrile and Subcutaneous Angiotensin II Infusion
In This Article
Summary
This protocol provides a detailed, step-by-step procedure for the induction of thoracic aortic dissection in mice. Specifically, it includes the precise calculation of the required doses of β-aminopropionitrile and angiotensin II, the procedure for osmotic pump filling, and the implantation technique of the osmotic pump.
Abstract
Thoracic aortic dissection (TAD) is a highly fatal cardiovascular disease that lacks efficient medical treatment. Replication of animal models of TAD pathophysiology is essential for studying the intrinsic mechanisms of TAD. The widely used TAD model induced by β-aminopropionitrile (BAPN, an irreversible and orally active lysyl oxidase inhibitor) in mice has the limitation of an inconsistent success rate. This protocol describes in detail a reported modified murine model of TAD induced by oral BAPN combined with subcutaneous angiotensin II (Ang II) infusion. After four weeks of BAPN administration followed by 24 h of Ang II infusion, a murine model with characteristics similar to human TAD was reliably induced, and the success rate of TAD model construction was significantly improved. Oral BAPN inhibits the cross-linking of elastin and collagen, resulting in the destruction of the aortic wall structure and inducing aortic dilation and dissection formation to a certain extent. The subsequent induction of Ang II further exacerbates the degeneration of the aortic wall, thereby promoting the occurrence of TAD. Consequently, the combination of BAPN and Ang II represents a refined approach to constructing a murine TAD model, offering a valuable tool to explore the pathogenesis and potential therapeutic approaches for TAD.
Introduction
Thoracic aortic dissection (TAD) is a serious aortic disease caused by an intimal tear due to bleeding within the wall of the thoracic aorta, resulting in separation of the aortic wall layers, blood entering the media of the aortic wall, forming a false lumen, and causing pressure on the true lumen1,2,3. Epidemiologic studies suggest that the incidence of TAD is between 7 and 9 cases per 100,000 people per year4. At present, it is believed that the pathogenesis of TAD is caused by the abnormal structure and hemodynamics of the aortic media, and factors such as hypertension, dyslipidemia, and hereditary vascular disease increase the risk of TAD5. Surgical intervention remains the primary treatment option for TAD. However, due to the high perioperative risks, exploring the pathogenesis of TAD and early intervention methods to delay its progression is of significant importance for improving the prognosis of TAD. As it is very difficult to obtain human samples and perform experiments directly in humans, it is necessary to establish animal models of TAD that mimic the characteristics of human TAD.
Over the past few decades, many animal models of aortic aneurysm (AA) have been widely reported. However, there are still few studies on the establishment of TAD models; some researchers have even considered TAD to be a byproduct of the AA animal model6. In fact, given that TAD results from an initial intimal tear of the thoracic aorta followed by rapid expansion of the false lumen, this significant difference in mechanism distinguishes TAD from aortic aneurysm7. To date, β-aminopropionitrile (BAPN)-induced rodent aortic dissection is the most used model of TAD. BAPN, a specific and irreversible inhibitor of lysyl oxidase, inhibits the cross-linking of elastic fibers and collagen fibers in the aortic wall, and is widely used in animal models of aortic dissection8,9,10. In most cases, BAPN has been added to the drinking water of mice to construct TAD models, and a combination of BAPN and angiotensin II (Ang II) via osmotic pump has been reported to construct TAD models11,12. However, these methods for building TAD models are not described in detail. Because of differences in mouse strains, BAPN administration, and the concentration and duration of Ang II, the incidence and extent of TAD lesions have been unstable across different experiments. Therefore, there is an urgent need for a stable method to construct mouse TAD models.
Here, this protocol describes in detail, step by step, a simple and highly successful method using a combination of BAPN-supplemented water and Ang II osmotic pump for constructing a mouse TAD model. This protocol is applicable to most labs and is easy to learn, allowing even researchers with no experience in mouse model construction to perform it consistently.
Protocol
Animal protocols were approved by the Institutional Animal Care and Use Committee of Tianjin Medical University (Approval Number TMUaMEC 2022036). Three-week-old C57BL/6J male mice were used in this study. Details of the reagents and equipment used are listed in the Table of Materials.
1. Animal maintenance and grouping
- Raise the mice on standard maintenance chow. Use three-week-old mice for this study.
- Randomly assign the mice to the control group (Control), the oral BAPN group (BAPN), the oral BAPN and saline infusion group (BAPN + Saline), and the oral BAPN and Ang II infusion group (BAPN + Ang II) (Figure 1). Provide the Control group mice with normal drinking water.
- Provide the BAPN group mice with drinking water supplemented with BAPN at a dose of 1 mg/g/day for 4 weeks. Infuse the mice in the BAPN + Ang II group with Ang II (1 ng/g/min) and the mice in the BAPN + Saline group with an equivalent amount of saline for 24 h after 4 weeks of oral BAPN administration.
2. Preparation for BAPN-supplemented drinking water
- Provide the mice with BAPN-supplemented drinking water for 4 consecutive weeks after 3 days of adaptive feeding, setting 1 day as an induction cycle.
- Weigh the mice in each cage to calculate the amount of BAPN required in the drinking water.
- Record the water volume (vol) of each cage over the past day.
- To ensure adequate water intake, use 1.3 times the daily water volume (1.3 x vol) as the amount of water for an induction cycle. Accordingly, dissolve 1.3 times the required weight of BAPN in the calculated volume of drinking water. Detailed calculation method and an example are shown in Table 1.
NOTE: Mice at this age are still in a period of rapid growth. It is recommended that the water intake volume of the mice be recorded daily throughout the model induction period and that the BAPN-supplemented drinking water in the cage be changed every day. To prevent the decomposition of BAPN, wrap the bottle in aluminum foil to protect it from light.
3. Calculation of Ang II mass
- Weigh each mouse and calculate the Ang II mass required for the experiment based on the maximum body weight.
- Use the calculation template (Table 2) to calculate the Ang II mass needed for the experiment.
- Calculate the mass of Ang II required for 130 µL of Ang II solution per mouse, since each pump requires approximately 100 µL.
- Use the calculation template (Table 3) to calculate the filling volume of Ang II solution and saline needed for the experiment.
4. Ang II dissolution
- Store lyophilized Ang II powder in a sealed vial at -80 °C. To prevent moisture condensation, allow the Ang II to equilibrate to room temperature and then centrifuge it before opening.
- Weigh the calculated amount of Ang II in a sterile microtube using an analytical balance.
- Add the calculated volume of normal saline into the microtube containing the lyophilized Ang II and vortex thoroughly until fully dissolved.
- Prepare the required Ang II solution separately for each mouse on a clean bench based on body weight. Turn the microtube upside down to ensure the solution is well-mixed.
5. Osmotic pump filling
- Obtain the two parts of the osmotic pump from the package and prepare the pump using sterile instruments to avoid the risk of infection.
- Weigh each pump, including the pump body and flow moderator, with an analytical balance. Record the data and use it to calculate the filling ratio.
- Attach the filling tube to a freshly opened 1 mL sterile syringe and carefully aspirate the prepared Ang II solution described above, taking care not to aspirate air bubbles.
- Hold the filling tube in the upward position and carefully remove any air bubbles from the syringe. Maintain this position to prevent air bubbles from entering the filling tube.
- Gently insert the end of the filling tube into the opening at the top of the osmotic pump until it cannot be inserted any further.
- Hold the osmotic pump upright and slowly squeeze the syringe plunger. Once the Ang II solution appears at the outlet, stop immediately and carefully remove the filling tube.
- Carefully and slowly insert the flow moderator into the opening of the osmotic pump until no gap is visible between the flow moderator and the top of the pump. Wipe off any excess Ang II solution with sterile absorbent paper.
- Weigh the loaded pump on an analytical balance and record it. The difference in pump weight before and after infusion is the mass of the loaded Ang II solution.
- Calculate the filling rate using the following formula:
Filling rate = (mass of filled pump - mass of empty pump) / (standard volume) x 100%
NOTE: The fill volume should be more than 90% of the standard volume indicated on the instruction sheet. If so, the fill is successful. If not, the pump should be drained and refilled. - Place the filled pumps in sterile saline at 37 °C with the moderator head up for at least 6 h until implantation.
6. Surgical procedure for pump implantation
- Autoclave all surgical instruments, including scissors, hemostats, tweezers, needle forceps, and suture needles, 24 h prior to pump implantation.
- Place the mouse in an anesthetic induction chamber with 1.5%-2% isoflurane at a flow rate of 2 L/min and stabilize the mouse for 2 min. Shave the mouse hair in an area of about 2 cm × 1 cm on the mid-scapular skin.
NOTE: Because the mouse's response to isoflurane is variable, the concentration may need to be adjusted to maintain stable anesthesia. - Place the mouse in a prone position with the nose in the nose cone connected to the isoflurane anesthetic machine. Apply veterinary ointment to the eyes to prevent dryness while under anesthesia. Ensure that the mouse does not respond to painful stimuli before and during pump implantation.
- Disinfect the dorsal skin three times with medical iodophor.
- Carefully make a transverse incision of about 1 cm on the skin using a scalpel.
- Gently grasp the incisal margin with curved forceps and bluntly dissect the subcutaneous tissue with another pair of curved forceps to create a pocket for the pump. Ensure the pocket is large enough to allow the pump to move freely.
- Insert the filled pump into the subcutaneous pocket with the flow moderator head positioned toward the caudal end of the mouse. Leave sufficient space to close the incision and avoid overstretching the skin.
- Once the pump has been implanted, neatly align the incisal margin and close the skin with 6-0 non-absorbable sutures.
NOTE: Carefully inspect the incision site to ensure that the wound is completely closed and that the pump is not pressing directly against the incision. - Clean the incision again with iodophor and apply 5% lidocaine anesthetic gel topically using a sterile cotton swab. Turn the isoflurane off (0%).
7. Postoperative animal care
- Monitor mice closely after pump implantation and place them in a recovery cage along with an electric heated blanket.
- Allow the mice to recover alone in a warm cage for at least 20 min until they wake up, and then return them to their original housing cage.
- Observe the mice hourly for the first 6 h after surgery. For the next 18 h, observe the mice at 6-h intervals. Collect samples immediately if mice are in a moribund state or die during Ang II administration.
8. Harvesting, fixing, cleaning, and imaging of aortas
- Anesthetize mice from all experimental groups with isoflurane and then sacrifice them by cervical dislocation at the end of the study.
- Place the mice in a supine position and secure them on a mouse plate. Make a lower abdominal incision and extend it across the chest wall until the thoracic and abdominal cavities are fully exposed.
- Immediately make a small incision in the right atrium, insert a sterile syringe into the left ventricle, and slowly inject approximately 10 mL of ice-cold phosphate-buffered saline (PBS) through the left ventricle until the lungs and liver turn white.
- Subsequently, perform resection of the lungs, liver, spleen, and intestines. Fully expose the entire aorta and measure the maximum diameter of the thoracic aorta using a digital caliper. Dissect the aorta from the heart and transect all arterial branches and common iliac arteries to harvest the entire aorta.
- Image the aorta with a digital camera, preserve it in 4% paraformaldehyde for 24-48 h, and embed it in paraffin for sectioning.
NOTE: Take care to avoid injury to the intestinal tract, as it may interfere with tissue analysis. - Cut aortic paraffin sections at a thickness of 3-5 µm using a microtome, then perform dewaxing and hydration. Stain the sections with hematoxylin and eosin (H&E) and Elastic-Van Gieson (EVG) using the respective staining kits according to the manufacturer's instructions.
Results
A total of 70 male C57BL/6J mice, aged 3 weeks, were included in this study and randomly assigned to four groups: Control (n = 10), BAPN (n = 20), BAPN + Saline (n = 20), and BAPN + Ang II (n = 20). In the BAPN group, 11 out of 20 mice developed thoracic aortic dissection (TAD) 28 days after BAPN administration, with 4 mice dying from aortic rupture. In the BAPN + Saline group, 12 out of 20 mice developed TAD, with 4 deaths due to rupture. Notably, in the BAPN + Ang II group, all 20 mice developed TAD, and 7 of them died from aortic rupture. No TAD formation was observed in the Control group (Figure 2A). Representative aortic images from each group are shown in Figure 2B.
The average maximum aortic diameters were 1.00 ± 0.09 mm (Control), 2.57 ± 0.22 mm (BAPN), 2.57 ± 0.33 mm (BAPN + Saline), and 2.78 ± 0.23 mm (BAPN + Ang II) (Figure 2C). Compared to the Control group, all three model groups showed significantly increased maximum aortic diameters; however, no significant differences were observed among the model groups themselves.
Histological analysis using H&E staining revealed thickened aortic walls and marked inflammatory cell infiltration in the BAPN, BAPN + Saline, and BAPN + Ang II groups, relative to the Control group. EVG staining further demonstrated fragmentation and loss of elastic fibers in these groups (Figure 3).

Figure 1: Schematic protocol for thoracic aortic dissection induction. Aortic dissection was induced in 3-week-old C57BL/6J mice through oral administration of β-aminopropionitrile (BAPN) or subsequent infusion of angiotensin II (Ang II). Mice in the Control and BAPN groups received normal drinking water or BAPN-supplemented drinking water (1 mg/g/day) for 4 weeks, respectively. Mice in the BAPN + Ang II group or the BAPN + saline group were infused with Ang II (1 ng/g/min) or an equivalent volume of saline for 24 h after completing four weeks of BAPN treatment. Please click here to view a larger version of this figure.
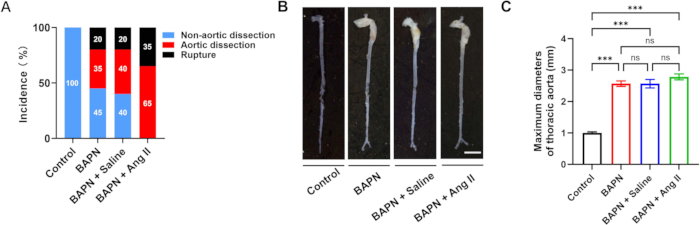
Figure 2: Aortic dissection incidence and morphology across groups. (A) Rates of aortic dissection and rupture in each group. (B) Representative gross morphology of aortas from each group; scale bar = 5 mm. (C) Quantification of maximal aortic diameters in mice with aortic dissection (n = 6 per group); ***p < 0.001. Please click here to view a larger version of this figure.
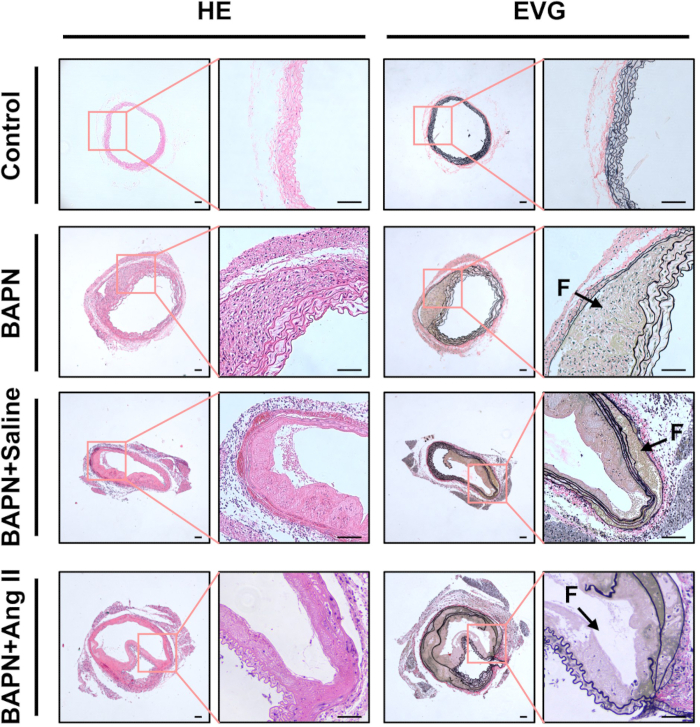
Figure 3: Histological characterization of dissected aortas. Representative hematoxylin and eosin (HE) and elastin van Gieson (EVG) stained sections of aortic tissue from each group. Black arrows indicate false lumens (F); scale bar = 100 µm. Please click here to view a larger version of this figure.
| Calculation of BAPN Amount | |
| Dose | 1 mg/g/d |
| Total body weight | x g |
| Dosage for an induction cycle | y mg = 1.3 x (x g x 1 mg/g/d x1 d) |
| Calculation of Water Volume | |
| Water intake (over the past day) | a mL |
| Water volume for an induction cycle | b mL = 1.3 x a mL |
Table 1: Calculation of BAPN dosage and water volume. The amount of BAPN required was based on the total body weight of mice per cage and the average daily water intake. To ensure adequate intake, the calculated water volume was multiplied by a factor of 1.3. For example, for four mice with a total body weight of 72.1 g and a daily water intake of 10.9 mL, 93.7 mg BAPN was dissolved in 14.17 mL of drinking water for one induction cycle.
| Calculation of Ang II Amount | ||
| General Condition | Dose | 1 ng/g/min |
| Body weight (Heaviest) | x g | |
| Release rate | 1 μL/h | |
| Number of mice | b | |
| Per mouse | Dosage per hour | y ng/h = x g × 60 min × 1 ng/g/min |
| Solution concentration | z ng/μL = (y ng/h) / (1 μL/h) | |
| Required solution volume per mouse | 130 μL | |
| Required Ang II mass per mouse | a ng = z ng/μL × 130 μL | |
| All mouse | Total Ang II requirement | m mg = a ng x 10-6 x b |
| Solute volume | n μL = 130 μL x b | |
Table 2: Calculation of Ang II quantity for osmotic pump infusion. The required Ang II mass was calculated to achieve a delivery rate of 1 ng/g/min for each mouse, assuming 130 µL of solution per pump. For example, for a 23.5 g mouse, the required solution concentration was 1410 ng/µL, resulting in a total Ang II mass of 1.833 mg for 10 mice. This amount was dissolved in 1300 µL of saline.
| Calculation of Filling Volume | ||||
| Body weight | Ang II Volume | Saline Volume | Total Volume | |
| Mouse 1# | 23.5 g | 100 μL | 0 μL | 100 μL |
| Mouse 2# | x g | a μL | b μL | 100 μL |
Table 3: Calculation of Ang II and saline filling volumes per mouse. Filling volumes were adjusted according to individual mouse body weight, with a maximum of 100 µL per pump. For example, a mouse weighing 22 g received 93.6 µL of Ang II solution, and the remaining 6.4 µL was filled with saline to reach the total 100 µL volume.
Discussion
Due to the limited understanding of life-threatening thoracic aortic dissection (TAD), the establishment of stable animal models is essential for exploring the molecular mechanisms underlying TAD onset and progression. β-Aminopropionitrile (BAPN), a lysyl oxidase inhibitor, is widely used in rodent models of TAD because it disrupts the cross-linking of collagen and elastin, thereby weakening the aortic wall and increasing its susceptibility to mechanical stress13. However, BAPN administration alone often results in inconsistent TAD incidence across studies.
As a lysyl oxidase inhibitor, BAPN irreversibly inhibits the cross-linking of elastin and collagen10. It is generally believed that during the juvenile phase, the cross-linking of these extracellular matrix components is still ongoing14. Therefore, administering BAPN during this critical developmental window may be particularly effective at disrupting matrix maturation, increasing the likelihood of successful TAD induction. Several studies have shown that BAPN alone can induce TAD in juvenile mice, although the reported incidence varies widely, ranging from 9% to 91% following 4 weeks of BAPN administration12,15,16.
Notably, the onset of TAD in humans has shown a trend toward younger age in recent decades, with some studies indicating that the average age of aortic complications occurs between 30 and 40 years17,18. Since BAPN induces TAD in 3- to 4-week-old mice by impairing elastin and collagen cross-linking during extracellular matrix development, this model may better reflect the pathophysiology and molecular features of early-onset TAD in humans19.
In contrast, BAPN alone is insufficient to induce TAD in adult mice9,20. To address this, various studies have combined BAPN with additional interventions. For instance, co-administration of NG-nitro-L-arginine methyl ester (L-NAME), BAPN, and angiotensin II (Ang II) has been shown to induce TAD in adult mice21. Among these, the combination of BAPN and Ang II is the most commonly used strategy to enhance TAD incidence. Ren et al. reported a 100% incidence of TAD when Ang II was infused for 24 h following a 4-week BAPN regimen, a result consistent with the findings of this study12. Furthermore, Ang II administration has been associated with dose-dependent mortality rates of 14%, 39%, and 67% after 12 h, 24 h, and 48 h of infusion, respectively22, which also aligns with the mortality rates observed in our experimental model.
There are several modes of BAPN administration, including delivery via drinking water, osmotic pumps, gastric tube, diet, and intraperitoneal injection21,22,23,24. Among these, administration through drinking water is the most commonly used method for juvenile mice. Osmotic pumps, on the other hand, are well-established for providing a consistent and sustained release of compounds and are frequently used for Ang II infusion in mouse models of aortic aneurysms25. Although subcutaneous infusion via pumps may be considered an optimal method for BAPN delivery, the solubility of BAPN and the capacity of the pump limit its feasibility. The maximum solubility of BAPN in water is approximately 50 mg/mL, which is insufficient to meet the concentration requirements for effective pump-based delivery. Therefore, a highly concentrated BAPN solution would be necessary. Similar to the present study, recent protocols have adopted a combined approach-administering BAPN in drinking water and delivering Ang II via osmotic pumps26. This method appears to be optimal for delivering BAPN to juvenile mice.
Currently, there is no standardized consensus in the literature regarding the optimal dose and duration of BAPN administration. While many studies have used a dose of 1 mg/g/day when BAPN is delivered through drinking water, others have described BAPN concentrations in terms of 1-3 mg/mL or 0.2% to 0.6% (wt/vol)27,28,29,30,31. The addition of BAPN to drinking water can alter the amount of water consumed by mice, and water intake may not correlate linearly with body weight. Consequently, a fixed BAPN concentration may result in variability in the actual BAPN intake per mouse. To address this, the current study adjusted the BAPN-supplemented drinking water daily based on both body weight and water intake, aiming to maintain a consistent dose of 1 mg/g/day.
This protocol, however, has several limitations. First, it lacks baseline data on the incidence and pathological progression of TAD at intermediate time points. Second, only male mice were used in this study. While TAD is more prevalent in males, females are reported to have worse outcomes, including higher mortality and reduced long-term survival following surgical treatment32,33. Interestingly, some studies have observed a lower incidence of aortic dissection in female mice treated with BAPN and Ang II26,34, which warrants further investigation. Third, the initiation of TAD induction at 3 weeks of age (analogous to human adolescence) may not fully capture the pathophysiological mechanisms underlying adult-onset TAD. Lastly, group housing during the BAPN administration period (4 mice per cage) introduces variability in individual water intake, which may contribute to differences in BAPN exposure and symptom severity.
In conclusion, this protocol outlines a stable, high-incidence, and reproducible mouse model of TAD that closely mimics the pathological features of human TAD. Owing to its simplicity and reliability, this model offers valuable utility for investigating the molecular mechanisms underlying TAD onset and progression, as well as for evaluating potential therapeutic strategies.
Disclosures
The authors of this manuscript have no conflicts of interest to declare.
Acknowledgements
This work was supported by a grant from the National Natural Science Foundation of China (82370299) and the Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-060B).
Materials
| Name | Company | Catalog Number | Comments |
| 3-Aminopropionitrile Fumarate salt | Sigma-Aldrich | A3134 | |
| Analytical balance | Radwag | AS 220.R2 | |
| Anesthesia Machine | Shanghai Renyi Biological Technology Co. Ltd. | MSS-3 | |
| Angiotensin II | MCE | HY-13948 | |
| C57BL/6J Male Mice | GemPharmatech | N000013 | |
| Chow Diet | Sibeifu Beijing Biotechnology Co. Ltd | SPF-F02-002 | |
| Electrothermal constant temperature water tank | Yiheng Technical Co. Ltd. | DK-8D | |
| EVG Staining Kit | Solarbio | G1590 | |
| GraphPad Prism | Graphpad | Ver 10.0.2 | |
| H&E Staining Kit | Servicebio | G1076 | |
| Hemostat | Shinva Medical Instrument Co. Ltd. | ZH240RN | |
| Isoflurane | RWD | R510-22-10 | |
| Microtube | Axygen Scientific, Inc. | MCT-150-C | |
| Needle forcep | Shinva Medical Instrument Co. Ltd. | ZM234R/RN/RB | |
| Osmotic pump | Alzet | 1003D | |
| Paraformaldehyde | Servicebio | G1101 | |
| PBS, 1x | Servicebio | G4202 | |
| Saline | Servicebio | G4702 | |
| Scalpel | Shinva Medical Instrument Co. Ltd. | ZB084R/RN | |
| Scissor | Shinva Medical Instrument Co. Ltd. | ZC480RN/RB/RNj/RNh | |
| Stereo microscope | Leica | EZ4 | |
| Suture | Jinhuan Medical Supplies Co. Ltd. | F604 | |
| Tweezer | Shinva Medical Instrument Co. Ltd. | ZO022RB |
References
- Erbel, R. et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases:Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. Eur Heart J. 35(41), 2873-2926 (2014).
- Bossone, E., Eagle, K. A. Epidemiology and management of aortic disease: Aortic aneurysms and acute aortic syndromes. Nat Rev Cardiol. 18 (5), 331-348 (2021).
- Carrel, T., Sundt, T. M., Von Kodolitsch, Y., Czerny, M. Acute aortic dissection. Lancet. 401 (10378), 773-788 (2023).
- Goldfinger, J. Z. et al. Thoracic aortic aneurysm and dissection. J Am Coll Cardiol. 64 (16), 1725-1739 (2014).
- Zhou, Z., Cecchi, A. C., Prakash, S. K., Milewicz, D. M. Risk factors for thoracic aortic dissection. Genes. 13 (10), 1814 (2022).
- Saraff, K., Babamusta, F., Cassis, L. A., Daugherty, A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin II-infused, apolipoprotein E-deficient mice. Arterioscler. Thromb Vasc Biol. 23 (9), 1621-1626 (2003).
- Hameed, I., Cifu, A. S., Vallabhajosyula, P. Management of thoracic aortic dissection. JAMA. 329 (9), 756-757 (2023).
- Liu, X. et al. Single-cell RNA sequencing identifies an IL1RN(+)/TREM1(+) macrophage subpopulation as a cellular target for mitigating the progression of thoracic aortic aneurysm and dissection. Cell Discov. 8 (1), 11 (2022).
- Kanematsu, Y. et al. Pharmacologically induced thoracic and abdominal aortic aneurysms in mice. Hypertension. 55(5), 1267-1274 (2010).
- Zheng, H. Q. et al. Induction of thoracic aortic dissection: A mini-review of β-aminopropionitrile-related mouse models. J Zhejiang Univ Sci B. 21 (8), 603-610 (2020).
- Hatipoglu, O. F. et al. Deficiency of CD44 prevents thoracic aortic dissection in a murine model. Sci Rep. 10 (1), 6869 (2020).
- Ren, W. et al. β-aminopropionitrile monofumarate induces thoracic aortic dissection in C57BL/6 mice. Sci Rep. 6, 28149 (2016).
- Wawzonek, S., Ponseti, I. V., Shepard, R. S., Wiedenmann, L. G. Epiphyseal plate lesions, degenerative arthritis, and dissecting aneurysm of the aorta produced by aminonitriles. Science. 121 (3133), 63-65 (1955).
- Fyfe, F. W., Gillman, T., Oneson, I. B. A combined quantitative chemical, light, and electron microscope study of aortic development in normal and nitrile-treated mice. Ann N Y Acad Sci. 149 (2), 591-627 (1968).
- Zhang, Y. et al. S-nitrosylation of septin2 exacerbates aortic aneurysm and dissection by coupling the TIAM1-RAC1 axis in macrophages. Circulation. 149 (24), 1903-1920 (2024).
- Pan, L. et al. Legumain is an endogenous modulator of integrin αvβ3 triggering vascular degeneration, dissection, and rupture. Circulation. 145 (9), 659-674 (2022).
- Groth, K. A. et al. Aortic events in a nationwide Marfan syndrome cohort. Clin Res Cardiol. 106 (2), 105-112 (2017).
- Eagleton, M. J. Arterial complications of vascular Ehlers-Danlos syndrome. J Vasc Surg. 64 (6), 1869-1880 (2016).
- Sawada, H., Beckner, Z. A., Ito, S., Daugherty, A., Lu, H. S. β-aminopropionitrile-induced aortic aneurysm and dissection in mice. JVS Vasc Sci. 3, 64-72 (2022).
- Franklin, M. K. et al. β-aminopropionitrile induces distinct pathologies in the ascending and descending thoracic aortic regions of mice. Arterioscler Thromb Vasc Biol. 44 (7), 1555-1569 (2024).
- Izawa-Ishizawa, Y. et al. Development of a novel aortic dissection mouse model and evaluation of drug efficacy using in-vivo assays and database analyses. J Hypertens. 37 (1), 73-83 (2019).
- Anzai, A. et al. Adventitial CXCL1/G-CSF expression in response to acute aortic dissection triggers local neutrophil recruitment and activation leading to aortic rupture. Circ Res. 116 (4), 612-623 (2015).
- Nishida, N. et al. High salt intake worsens aortic dissection in mice: Involvement of IL-17A-dependent ECM metabolism. Arterioscler Thromb Vasc Biol. 40 (1), 189-205 (2020).
- Chen, J. Y. et al. Increased aortic stiffness and attenuated lysyl oxidase activity in obesity. Arterioscler Thromb Vasc Biol. 33 (4), 839-846 (2013).
- Lu, H. et al. Subcutaneous angiotensin II infusion using osmotic pumps induces aortic aneurysms in mice. J Vis Exp. 103, e53191 (2015).
- Qi, X. et al. A validated mouse model capable of recapitulating the protective effects of female sex hormones on ascending aortic aneurysms and dissections. Physiol Rep. 8 (22), e14631 (2020).
- Obama, T. et al. Epidermal growth factor receptor inhibitor protects against abdominal aortic aneurysm in a mouse model. Clin Sci (Lond.). 128 (9), 559-565 (2015).
- Aicher, B. O. et al. Moderate aerobic exercise prevents matrix degradation and death in a mouse model of aortic dissection and aneurysm. Am J Physiol Heart Circ Physiol. 320 (5), H1786-H1801 (2021).
- Aicher, B. O. et al. Quantitative micro-CT analysis of aortopathy in a mouse model of β-aminopropionitrile-induced aortic aneurysm and dissection. J Vis Exp. 137, e57589 (2018).
- Ohno-Urabe, S. et al. Authentication of in situ measurements for thoracic aortic aneurysms in mice. Arterioscler Thromb Vasc Biol. 41 (6), 2117-2119 (2021).
- Sawada, H. et al. Ultrasound monitoring of descending aortic aneurysms and dissections in mice. Arterioscler Thromb Vasc Biol. 40 (10), 2557-2559 (2020).
- Deery, S. E. et al. Female sex independently predicts mortality after thoracic endovascular aortic repair for intact descending thoracic aortic aneurysms. J Vasc Surg. 66 (1), 2-8 (2017).
- Rylski, B. et al. Gender-related differences in patients with acute aortic dissection type A. J Thorac Cardiovasc Surg. 162 (2), 528-535.e521 (2021).
- Fashandi, A. Z. et al. Female mice exhibit abdominal aortic aneurysm protection in an established rupture model. J Surg Res. 247, 387-396 (2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved