Method Article
Two-dimensional Porcine Intestinal Organoids Reflecting the Physiological Properties of Native Gut
In This Article
Summary
This study outlines a protocol for generating 2D monolayers of porcine organoids derived from the small and large intestines. The growth of these monolayers is marked by increasing TEER values, indicating robust epithelial integrity. Additionally, these monolayers exhibit physiological secretory responses in Ussing chamber experiments following the application of forskolin.
Abstract
The gastrointestinal tract (GIT) serves both in the digestion of food and the uptake of nutrients but also as a protective barrier against pathogens. Traditionally, research in this area has relied on animal experiments, but there's a growing demand for alternative methods that adhere to the 3R principles-replace, reduce, and refine. Porcine organoids have emerged as a promising tool, offering a more accurate in vitro replication of the in vivo conditions than traditional cell models. One major challenge with intestinal organoids is their inward-facing apical surface and outward-facing basolateral surface. This limitation can be overcome by creating two-dimensional (2D) organoid layers on transwell inserts (from here on referred to as insert(s)), providing access to both surfaces. In this study, we successfully developed two-dimensional cultures of porcine jejunum and colon organoids. The cultivation process involves two key phases: First, the formation of a cellular monolayer, followed by the differentiation of the cells using tailored media. Cellular growth is tracked by measuring transepithelial electrical resistance, which stabilizes by day 8 for colon organoids and day 16 for jejunum organoids. After a 2-day differentiation phase, the epithelium is ready for analysis. To quantify and track active electrogenic transport processes, such as chloride secretion, we employ the Ussing chamber technique. This method allows for real-time measurement and detailed characterization of epithelial transport processes. This innovative in vitro model, combined with established techniques like the Ussing chamber, provides a robust platform for physiologically characterizing the porcine GIT within the 3R framework. It also opens opportunities for investigating pathophysiological mechanisms and developing potential therapeutic strategies.
Introduction
The GIT plays a central role in digestion, nutrient absorption, and waste excretion through feces1. Additionally, it functions as a barrier against pathogens, a role supported by a diverse cellular composition, including stem cells, mucus-producing goblet cells, enteroendocrine cells, and absorptive enterocytes2. Intestinal homeostasis can be disrupted by various factors, such as bacterial infections3 or inflammatory processes4, leading to severe consequences for the organism, such as malabsorption, diarrhea, or even death5. Investigating such pathophysiological scenarios is commonly done using laboratory animals or, in accordance with the 3R principle6, cell cultures derived from various species. Accurate prediction and transferability of results are crucial when employing species-specific models7. Despite this need, there is a notable lack of pig-derived cell cultures that adequately replicate the complexity and functionality of the intestinal tract.
To address this challenge, also relevant to other species, three-dimensional (3D) organoids have been developed in an attempt to replicate the physiological complexity of the GIT8. Initially, organoids were created from human and mice intestines; to date, porcine organoids from juvenile and adult pigs have also been successfully developed and cultivated9,10. Since their inception, these porcine organoids have been utilized in several studies, primarily focusing on intestinal infections11,12,13,14. Research aimed at characterizing physiological properties, such as nutrient transport or secretory processes, remains limited15. This may be due to the orientation of intestinal organoids, with the apical surface facing inward and the basolateral side outward, limiting accessibility to the apical surface. This limitation was addressed by successfully cultivating porcine organoids in a two-dimensional format16, a method that has been further advanced through the use of frozen tissue to generate them17.
The 2D cultivation of porcine organoids provides access to both sides of the epithelium, enabling the application of well-established methods to study transport processes across the epithelial layer. One such method is the Ussing chamber18, which allows real-time observation of electrogenic absorptive and secretory processes across the epithelium. Extensive use of this system has provided a comprehensive understanding of the porcine intestinal function in vivo, covering the entire intestinal axis. This includes studies on monosaccharide transport or transport of short-chain fatty acids or responses to secondary plant metabolites such as resveratrol that influence intestinal transport characteristics19,20,21,22,23,24. The substantial body of data from these studies facilitates direct comparisons between the well-characterized in vivo conditions and the in vitro environment of porcine organoids, enhancing our understanding of their physiological relevance.
In this study, we present a protocol for generating and cultivating 2D monolayers from 3D porcine organoids. Additionally, we detail the methodological approach for quantifying intestinal transport processes using the Ussing chamber technique. The protocol offers tools to study absorptive and secretory characteristics in vitro in jejunum and colon organoids, allowing for direct comparison with well-characterized in vivo conditions. Future applications of this protocol may include investigating the effects of pharmacological or toxicological substances, as well as exploring interactions between the epithelium and pathogens.
Protocol
For this protocol, two healthy pigs (Bentheim Blacked Pied pig; 1 male, 1 female; 4.5 months old; approximately 65 kg) were sacrificed by captive bolt shoot and bleeding. According to the Animal Protection Law, this (slaughter and removal of tissues) is not classified as an animal experiment but has to be announced to the animal welfare officer (registration no. TiHo-T-2023-15) of the University of Veterinary Medicine Hannover Foundation.
1. Coating of inserts
NOTE: All steps are carried out with sterile materials under a safety cabinet. All steps of the protocol are performed on ice if not stated otherwise.
- Thaw the frozen basement membrane on ice for at least 1 h at room temperature or overnight on the ice at 4 °C. Mix the basement membrane 1:40 (v/v) with ice-cold sterile phosphate-buffered saline (PBS) in a conical tube.
- Remove the sterile plates with inserts from the packaging. Add 200 µL of basement membrane mix to the apical compartment of each insert.
- Replace the lid of the well plate and incubate for at least 1.5 h at 37 °C, 5% CO2 in an incubator.
- Aspirate the solution carefully before seeding the cells. Ensure that the tip does not touch the membrane during aspiration.
2. Generation of 2D organoid monolayers
NOTE: Porcine colon organoids are generated and cultivated as described for porcine jejunal organoids25. After generation of 3D organoids these should be cultivated for at least 3-4 weeks while weekly passaging to ensure consistent growth. The number of cells within each dome containing 3D organoids, which are dissolved in the subsequent steps, is sufficient to cover a single transmembrane filter. Before monolayer generation, the 3D organoids were subjected to optical quality control to check the previous growth and possible contamination (Figure 1).
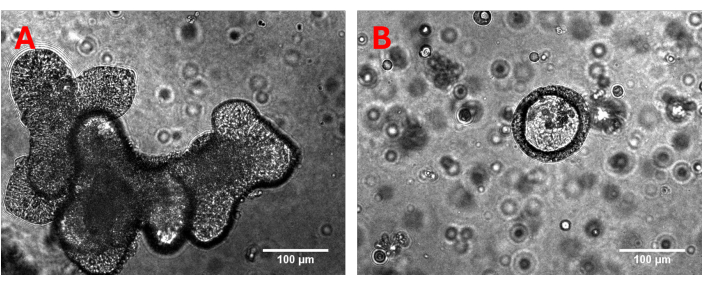
Figure 1: Representative 3D organoids. Three-dimensional (A) jejunum and (B) colon organoids are carefully examined under the microscope prior to generating monolayers. Special attention is given to assessing previous growth patterns, structural integrity, and the presence of any impurities or contamination. Please click here to view a larger version of this figure.
- Remove the organoid medium (Table 1) from the wells with 3D crypt organoids. Add 1 mL of ice-cold PBS per well and gently dissolve the basement membrane by pipetting up and down with a p1000 tip.
- Collect all dissolved organoids in a 15 mL tube pre-filled with 10 mL of ice-cold PBS. Centrifuge the tube at 250 x g, 10 min at 4 °C. Aspirate the supernatant.
- Resuspend pellet in 1 mL of warm (37 °C) 0.05 % (v/v) trypsin/EDTA (2 tubes can be pooled at this stage). Incubate for 5 min at 37 °C in a water bath and put directly on ice afterward to stop the reaction.
- Resuspend on ice 20x with p1000 tip, and another 15x with p1000 tip + p200 tip on top. Add 10 mL of ice-cold DMEM supplemented with 10% (v/v) fetal calf serum (FCS).
- Centrifuge tube at 1,000 x g, 10 min at 4 °C. Discard supernatant and resuspend pellet in 1 mL of monolayer medium (Table 1).
- Determine the number of living cells per mL using a Neubauer chamber according to the manufacturer's instructions.
- Remove the coating solution at this point from the apical compartment and replace it with 500 µL of warm (37 °C) monolayer medium. Add 3 mL of monolayer medium to the basolateral compartment.
- Determine the blank resistance (TEER) of each empty transwell insert, as described in detail in step 3. The time in which no medium is present in the apical chamber should be as short as possible to prevent the cells from drying out.
- Remove the apical monolayer medium and add for the jejunal 2D culture 2 x 105 epithelial cells and for the colonic 2D culture 1.5 x 105 epithelial cells in 500 µL of monolayer medium in the apical chamber of each transwell.
- Change medium and measure TEER every 2-3 days. Measure TEER before the medium is changed. Carefully aspirate the medium of the apical and basal compartment and replace it with 500 µL or 3 mL of fresh, warm (37 °C) medium.
- For jejunum organoids: on day 16, after seeding, change from monolayer medium to differentiation medium (Table 1). For colon organoids: change medium on day 7 to differentiation medium.
- Change the differentiation medium every day and perform TEER measurement during each medium change. On day 18 (for jejunal organoids) or day 9 (for colon organoids), after seeding, proceed with functional experiments.
3. Measurement of transepithelial electrical resistance (TEER)
NOTE: All measurements are performed under a safety cabinet to avoid contamination. Before seeding the cells, every empty coated transwell is measured to get individual blank values. The volt-ohm meter stores values on an introduced USB device.
- Clean the chopstick electrode with 70% (v/v) ethanol and allow the electrode to dry completely. Meanwhile, remove the plate from the incubator and place it under the cabinet.
- Introduce the short arm of the chopstick electrode to the apical compartment of the inserts and the long arm to the basolateral compartment of the insert. Avoid the contact of the short arm with the cell layer.
- Measure the transepithelial electrical resistance of each transwell. Allow the volt-ohm meter to equilibrate to ensure a stable measurement. Measure TEER values by clicking the Store button.
- Continue steps 3.2 and 3.3 with the remaining wells. After reaching the last well, the volt-ohm meter opens a request to store the data on a USB device. Click Save.
- Clean the electrode after each plate and at the end of the measurement and allow it to dry completely before storage.
- Subtract blank values determined before seeding the cells from the measured cellular values.
4. Electrophysiological transport studies using the Ussing chamber technique
NOTE: Determination of electrophysiological transport studies is performed by using a Ussing chamber consisting of two chamber compartments, which are divided by the epithelium. This chamber is connected to a voltage clamp by Ag/AgCl Electrodes. This technique allows the tracking of active electrogenic transport processes of the epithelium through the changes in the short-circuit current (Isc) induced by the voltage clamp as well as the tissue resistance (Rt) calculated by Ohm´s law. Isc and Rt are recorded every 6 s during the whole experiment. During the experiment the investigated tissue is aerated with carbogen and incubated with modified Krebs-Henseleit solutions to ensure viable conditions. Indomethacin (10 µM) is added to buffer solutions to inhibit prostaglandin synthesis26.
- Warm-up mucosal and serosal buffer solutions to 37 °C and aerate with carbogen. Assemble the single chambers using an empty insert for each individual chamber. Make sure the apical sides of the transwells are all facing in the same direction.
- Fill all chambers with 5 mL of pre-warmed mucosal buffer solution (Table 2). Connect all electrodes from the voltage clamp to the individual chambers according to the manufacturer's instructions. Make sure no gas bubbles are interfering with the electrodes to ensure correct measurement.
- Calibrate the Ussing chamber software with these conditions by clicking the Rf/dpI button in the voltage-clamp software. Resistance of all used empty inserts should be equal (~ 70 Ohm), and the current should be around 0 mV (± 5 mV). If this is not the case for some chambers, check for the proper placement of electrodes or bubbles in the system.
- Remove all electrodes and discard the used buffer solution. Open all individual chambers and remove the empty inserts. Make sure that the order of the individual chambers is maintained.
- Move the well plate with inserts from the incubator to the safety cabinet. Carefully aspirate the basolateral and apical medium.
- Wash the cells by adding 500 µL of warm (37 °C) mucosal buffer to the apical chamber and 3 mL of serosal buffer to the basolateral chamber. Aspirate the buffers and repeat 2x.
- Remove the inserts from the plate. Gently remove the supports of the inserts. Place the inserts into the Ussing chambers, and make sure the orientation of the inserts is the same as during the calibration phase. Assemble the individual chambers as displayed in Figure 2.
- Fill the chambers facing the basolateral side of the cells with 5 mL of serosal buffer (Table 2) and the chamber facing the apical chamber with 5 mL of mucosal buffer.
- Connect the electrodes and the aeration tubes to each individual chamber. Start measurement in the Ussing software. Allow equilibration for 15 min.
- Change the conditions from open circuit to short-circuit conditions. Equilibrate for 5 min to 10 min. Add 10 µM of forskolin to the serosal chamber.
NOTE: The addition of forskolin may be replaced by other agents/substances depending on individual intestinal segments and research questions. - After 15 min, either add more agents in the same manner as forskolin or stop the measurement. Remove aeration tubes and electrodes from individual chambers. Pour the buffer solutions of both chambers into a bowl.
- Disassemble the chambers and either discard the inserts or use them for follow-up analysis (e.g., immunohistochemistry or expression analysis)

Figure 2: Schematic structure of the Ussing chamber. Displayed are both chambers divided by the membrane with the grown 2D monolayer. Both chambers are aerated with carbogen; voltage and current are monitored by two electrodes per chamber. Please click here to view a larger version of this figure.
5. Analysis of data generated by the Ussing chamber setup
- Import the data generated by the voltage clamp software into a suitable spreadsheet editor.
- To determine basal Isc and Rt values, calculate the mean of 10 consecutive data points taken 10 min after initializing the short-circuit conditions. Each individual chamber serves as a technical replicate, while every passage of organoids serves as a biological replicate.
- To assess the effects of applied agents, calculate the mean of 10 consecutive data points immediately before the agent's addition. Compare this mean to the maximum (or minimum) value observed after the agent is applied. Data obtained is presented as mean ± standard deviation (SD).
Results
This protocol facilitates the reliable generation of porcine 2D monolayers by disaggregating 3D organoids derived from the jejunum and colon of pigs. Over a cultivation period of 16 days for jejunum organoids and 9 days for colon organoids, intact monolayers are formed. These monolayers can subsequently be used to assess electrogenic and physiological transport properties using the Ussing chamber technique.
After disintegrating the 3D organoids, single cells are seeded onto coated inserts. Cellular growth is monitored by measuring transepithelial electrical resistance (TEER) values during each medium change. This approach enables the tracking of both cell proliferation and the formation of an intact monolayer, which is essential for establishing a functional epithelium. Initially, the TEER values are close to baseline, but they progressively increase over the cultivation period, reaching a plateau at approximately 150 Ω*cm2 for jejunum organoids (Figure 3A) after 18 days and around 200 Ω*cm2 for colon organoids (Figure 3B) after 9 days of cultivation.
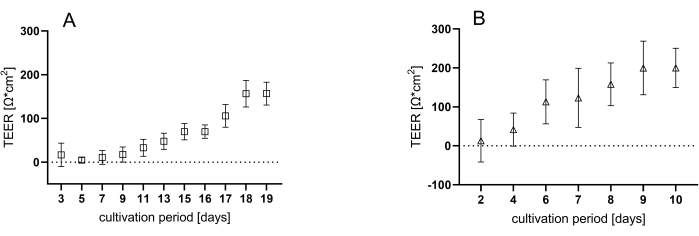
Figure 3: Measurement of TEER during the cultivation time. Representative values of three individual plates containing six wells each during the cultivation period of either (A) 18 days for jejunal or (B) 9 days for colonic organoids. Data shown are mean ± SD of three independent experiments. Please click here to view a larger version of this figure.
After the 2-day differentiation period, 2D organoids are mounted in Ussing chambers to monitor electrophysiological parameters such as Isc, which reflects ion transport, and Rt, which indicates tissue integrity. Both parameters are recorded every 6 s throughout the experiment. Following an initial equilibration period under open-circuit conditions, the system is switched to short-circuit conditions, allowing the measurement of active electrogenic transport processes across the epithelium. This transition is followed by another equilibration period, during which the basal values of the epithelium are established. Notably, the basal Isc (Figure 4A) of jejunum organoids is significantly lower (0.67 ± 0.36 µA/cm2) compared to colon organoids (7.12 ± 3.74 µA/cm2). In contrast, basal Rt values (Figure 4B) show no significant difference between the two organoid types, with values of approximately 175 Ω*cm2 for jejunum organoids and about 135 Ω*cm2 for colon organoids.

Figure 4: Basal electrophysiological values of colonic and jejunal organoids. Representative values of the Isc (A) and Rt (B) values of two-dimensional jejunal and colonic organoids determined by the Ussing chamber technique. Values were obtained 10 min after changing the conditions from open circuit to short-circuit by calculating the mean of 10 consecutive values. Data shown are mean ± SD of three independent experiments. Unpaired t-test; *** = p<0.001. Please click here to view a larger version of this figure.
The Ussing chamber technique is an essential tool for monitoring active electrogenic transport processes in epithelial tissues, which are crucial for validating and assessing the functionality of epithelial in vitro models. In this study, both jejunal and colonic organoids were treated with forskolin on the serosal side. This exposure led to a significant increase in Isc in the jejunal organoids, rising from 0.86 ± 4.59 µA/cm2 to 27.78 ± 2.27 µA/cm2 (Figure 5A). Similarly, the Isc in colonic organoids increased from 3.83 ± 2.25 µA/cm2 to 28.78 ± 1.06 µA/cm2 following forskolin application (Figure 5B).
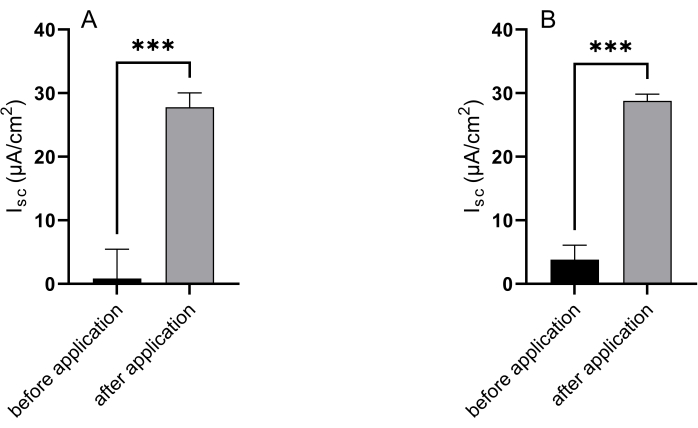
Figure 5: Epithelial response to forskolin simulation. Representative results of the Isc of (A) jejunal and (B) colonic organoids before and after application of forskolin to the serosal compartment. Basal values were obtained by calculating the mean of the last 10 values before the addition of forskolin, while values after application were the maximal response to the stimulating agent. Data shown are mean ± SD of three independent experiments. Unpaired t-test; * = p<0.05. Please click here to view a larger version of this figure.
Table 1: Medium compositions used for organoid cultivation. Please click here to download this Table.
Table 2: Buffer composition of the used Ussing buffers. Please click here to download this Table.
Discussion
This protocol describes a method for converting established porcine 3D organoids into single cells, which are then seeded onto transwell membranes to form an intact monolayer. This configuration grants access to the apical side of the cells, facilitating the use of Ussing chambers to monitor absorptive and secretory processes.
The initial and crucial step in this multi-step process is the precise disintegration of the 3D organoids. Achieving uniform seeding of the single cells is essential for the formation of a functional monolayer27. Therefore, careful monitoring of the disintegration process is necessary. Incubation with trypsin/EDTA solution should be adjusted accordingly - prolonged if cell clumps are observed or shortened if excessive cell death is noted during cell counting. It is important to note that variations in the size of the organoids, which may result from differences in user handling, can impact the disintegration process and should be taken into consideration.
The increasing TEER values, which reach a plateau phase at day 9 for the colon organoids or day 18 in the case of the jejunum organoids, indicate an intact monolayer28 as it has already been shown in well-established human cell culture systems of the intestinal tract29,30 and also in porcine organoids15,17. However, these publications show TEER values of more than 750 Ω*cm2 after 1-4 days15 or 500-2,500 Ω*cm2 after 3 days of cultivation17 which exceed values obtained in this study. These differences are presumably derived from different media compositions or seeding densities of the cells. Furthermore, these conditions may also influence cell proliferation and differentiation. While monolayers in previous studies developed monolayers within a few days15,16,17, our protocol results in a slower monolayer formation, followed by a subsequent differentiation phase. This extended timeline is likely due to specific cultivation conditions. The increasing TEER itself is facilitated by the increasing expression of expression of tight junction proteins such as claudins (e.g., claudin 2 and claudin 3) or occludin, leading to a higher interconnection of the individual cells31,32.
The experimental approach utilizes the Ussing chamber technique to monitor active transport processes across the epithelial layer. Notably, the basal Isc values differed between jejunal and colonic monolayers, with colonic monolayers displaying higher Isc values than their jejunal counterparts. This observation suggests that the variations between the two epithelia are likely to result from differing levels of electrogenic transport processes. These findings align with those observed in native porcine epithelium, highlighting the high degree of comparability of this model to the in vivo situation23. In contrast to the differences in Isc values, the Rt values, which are the reciprocal of tissue conductance, are comparable between both organoid types, although they remain higher than those recorded in native tissues19,23,33.
In summary, colon and jejunum organoids exhibit distinct TEER values, as well as differences in basal Isc and basal Rt, which determine the Ussing chamber setup. These differences align with findings from previous studies on native pigs small and large intestinal tissue23,34,35. The occurrence of similar differences in our in vitro system, as observed in vivo, further strengthens the comparability between the two conditions.
In addition to basal parameters, functional changes driven by active transport processes across the epithelium are crucial for establishing a functional intestinal model. In this study, we examined the effects of forskolin, which activates adenylate cyclase36, leading to elevated cyclic adenosine monophosphate (cAMP) levels. This, in turn, activates the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel, resulting in increased chloride secretion. Consequently, a significant rise in the Isc was observed in both jejunal and colonic monolayers. These results closely resemble the data obtained from native porcine tissue in these intestinal segments, underscoring the validity of the model20,22,23,37. Besides cAMP-dependent chloride secretion is present in both intestinal segments, segment-specific transport processes may be investigated in the Ussing chamber in future studies. These could include the absorption of monosaccharides such as glucose38 by the sodium-dependent glucose transporter 139 in the small intestine or the influence of the sodium transport by the amiloride-sensitive sodium channel in the large intestine40.
Overall, depending on the species from which the organoids are derived, and the specific method used for generating the 3D organoids, protocol modifications may be required. Furthermore, the growth of organoids may be influenced by whether they are generated from fresh tissue, as in this study, or from frozen crypts17. These adjustments could involve extending or shortening incubation and cultivation times at some stages of the protocol. The present study reveals differences in both cultivation parameters and experimental outcomes among the organoid types investigated. These findings indicate that organoids derived from different segments of the intestinal tract may require further adjustments and the protocol needs validation during the cultivation process depending on the abovementioned reasons.
Further limitations of this protocol include the need for more structural investigations, as it focuses solely on electro- and transport physiological properties. Additionally, careful handling of the organoids is crucial during both cultivation and Ussing chamber experiments due to their high vulnerability. For instance, excessive aeration in the Ussing chamber can cause cells to detach from the transwells and disrupt the monolayer due to shear stress, and this must be avoided.
In conclusion, the presented method enables the generation of porcine 2D monolayers characterized by increasing TEER values, tissue integrity, and functional capabilities, as demonstrated by the chloride secretion through the CFTR channels. By combining the relatively new organoid system - which is still undergoing refinement, particularly in the case of farm animal organoids - with the well-established Ussing chamber technique, we provide a crucial foundation for further validation and comparison with data obtained from native tissue. Additionally, this approach offers a valuable platform for addressing pathophysiological and pharmacological research questions.
Disclosures
We do not have any conflicts of interest to declare.
Acknowledgements
We thank the Federal Ministry of Food and Agriculture (BLE# 28N-2-071-00) for funding.
Materials
| Name | Company | Catalog Number | Comments |
| 24 well plate | SARSTEDT AG & Co. KG | 8,33,922 | |
| A83-01 | MedChemExpress, New Jersey, USA | HY-10432 | Store at -20 °C. Thaw when needed |
| accujet S | Brand GmbH + Co KG, Wertheim, Germany | 26351 | |
| Advanced DMEM/F12 Medium | Thermo Fisher Scientific, Waltham, USA | 12634010 | Store at 4 °C |
| B27 supplement | Thermo Fisher Scientific, Waltham, USA | 17504044 | Store at -20 °C. Thaw when needed |
| CaCl2.2 H2O | Merck KGaA, Darmstadt, Germany | C3306 | Store at room temperature |
| D(+)-Glucose (wasserfrei) | Merck KGaA, Darmstadt, Germany | 1.08337 | Store at room temperature |
| DAPT | MedChemExpress, New Jersey, USA | HY-13027 | Store at -20 °C. Thaw when needed |
| D-Mannitol | Merck KGaA, Darmstadt, Germany | M4125 | Store at room temperature |
| DMSO | Sigma-Aldrich, Schnelldorf, Germany | 154938 | Store at room temperature |
| Electrode-Set (AgCl/PtIr/Std.) | Scientific Instruments, Simmerath, Germany | #1316 | |
| Eppendorf Research plus | Eppendorf SE, Hamburg, Gemany | 3123000063 | |
| Eppendorf Research plus | Eppendorf SE, Hamburg, Gemany | 3123000047 | |
| EVOM3 Manual Epithelial Volt Ohm Meter | World precision instruments, Sarasota, USA | EVM-MT-03-01 | |
| FBS | Sigma-Aldrich, Schnelldorf, Germany | F7524 | Store at -20 °C. Thaw when needed |
| Forskolin | Sigma-Aldrich, Schnelldorf, Germany | F6886 | Store at -20 °C. Thaw when needed |
| gasprofi 1 SCS micro | WLD-TEC GmbH, Arsenhausen, Germany | 60,04,000 | |
| Gastrin 1 | MedChemExpress, New Jersey, USA | HY-P1097 | Store at -20 °C. Thaw when needed |
| Glutamax | Thermo Fisher Scientific, Waltham, USA | 35050061 | Store at 4 °C. |
| HCl | Sigma-Aldrich, Schnelldorf, Germany | 1090571000 | Store at room temperature |
| HEPES | Sigma-Aldrich, Schnelldorf, Germany | H0887 | Store at 4 °C |
| Herasafe 2025 Class II Biological Safety Cabinet | Thermo Fisher Scientific, Waltham, USA | 51033316 | |
| Incubator ICO105 | Memmert GmbH + Co.KG, Schwabach, Germany | 62,20,143 | |
| Indomethacin | Merck KGaA, Darmstadt, Germany | I7378 | Store at room temperature |
| KCl | Merck KGaA, Darmstadt, Germany | 1.04936 | Store at room temperature |
| L-Glutamin | Sigma-Aldrich, Schnelldorf, Germany | G7513 | Store at -20 °C. Thaw when needed |
| LWRN Supernatant | selfmade | Store at -20 °C. Thaw when needed. LWRN supplement is produced according to Miyoshi et al. (2012) | |
| Matrigel Basement Membrane Matrix, LDEV-free, 10 mL | Corning Incorporated - Life Sciences | 354234 | Store at -20 °C. Thaw carefully on ice when needed |
| Megafuge 1.OR | Heraeus Instruments, Osterode, Germany | 75003060 | |
| MgCl2.6 H2O | Merck KGaA, Darmstadt, Germany | 1.05833 | Store at room temperature |
| Na2HPO4.2H2O | Merck KGaA, Darmstadt, Germany | 1.06580 | Store at room temperature |
| N-Acetyl-L-cysteine | Sigma-Aldrich, Schnelldorf, Germany | A7250 | Store at -20 °C. Thaw when needed |
| NaCl | Merck KGaA, Darmstadt, Germany | 1.06404 | Store at room temperature |
| NaH2PO4.H2O | Merck KGaA, Darmstadt, Germany | 1.06346 | Store at room temperature |
| NaHCO3 | Merck KGaA, Darmstadt, Germany | 1.06329 | Store at room temperature |
| Neubauer improved chamber | Glaswarenfabrik Karl Hecht, Sondheim vor der Rhön, Germany | 40442712 | |
| Olympus IX70 iverted Microscope | Olympus Corporation, Hamburg, Germany | ||
| Pen/Strep | Thermo Fisher Scientific, Waltham, USA | 15140122 | Store at -20 °C. Thaw when needed |
| PolymyxinB | Sigma-Aldrich, Schnelldorf, Germany | P4932-1MU | Store at -20 °C. Thaw when needed |
| Primovert microscope stand with binocular phototube | Zeiss | 415510-1101-000 | |
| rm EGF | Prepotech, New Jersey, USA | 315-09 | Store at -20 °C. Thaw when needed |
| SB202190 | MedChemExpress, New Jersey, USA | HY-10295 | Store at -20 °C. Thaw when needed |
| Snapwell 3801 | Corning Incorporated - Life Sciences | 3801 | |
| Trypsin/EDTA | Thermo Fisher Scientific, Waltham, USA | 25300054 | |
| Ussing Base System | Scientific Instruments, Simmerath, Germany | #1317 | |
| Ussing Diffusion Chamber | Scientific Instruments, Simmerath, Germany | SKU 1307 | |
| Voltage/Current Clamp VCC6 | Scientific Instruments, Simmerath, Germany | SKU 1310 | |
| Y27632 | MedChemExpress, New Jersey, USA | HY-10583 | Store at -20 °C. Thaw when needed |
References
- Hornbuckle, W. E., Tennant, B. C. . Clinical Biochemistry of Domestic Animals. , 367-406 (1997).
- Peterson, L. W., Artis, D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 14 (3), 141-153 (2014).
- Pham, T. A., Lawley, T. D. Emerging insights on intestinal dysbiosis during bacterial infections. Curr Opin Microbiol. 17 (100), 67-74 (2014).
- Garrett, W. S., Gordon, J. I., Glimcher, L. H. Homeostasis and inflammation in the intestine. Cell. 140 (6), 859-870 (2010).
- Ternhag, A., Torner, A., Svensson, A., Ekdahl, K., Giesecke, J. Short- and long-term effects of bacterial gastrointestinal infections. Emerg Infect Dis. 14 (1), 143-148 (2008).
- Russell, W. M. S., Burch, R. L. . The principles of humane experimental technique. , (1959).
- Zietek, T., Boomgaarden, W. A. D., Rath, E. Drug screening, oral bioavailability and regulatory aspects: A need for human organoids. Pharmaceutics. 13 (8), 1280 (2021).
- Sato, T., et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 459 (7244), 262-265 (2009).
- Gonzalez, L. M., Williamson, I., Piedrahita, J. A., Blikslager, A. T., Magness, S. T. Cell lineage identification and stem cell culture in a porcine model for the study of intestinal epithelial regeneration. PLoS One. 8 (6), e66465 (2013).
- Khalil, H. A., et al. A novel culture system for adult porcine intestinal crypts. Cell Tissue Res. 365 (1), 123-134 (2016).
- Derricott, H., et al. Developing a 3D intestinal epithelium model for livestock species. Cell Tissue Res. 375 (2), 409-424 (2019).
- Li, L., et al. Porcine intestinal enteroids: a new model for studying enteric coronavirus porcine epidemic diarrhea virus infection and the host innate response. J Virol. 93 (5), e01682-e01718 (2019).
- Min, S., Kim, S., Cho, S. W. Gastrointestinal tract modeling using organoids engineered with cellular and microbiota niches. Exp Mol Med. 52 (2), 227-237 (2020).
- Yin, L., et al. Aminopeptidase N expression, not interferon responses, determines the intestinal segmental tropism of porcine deltacoronavirus. J Virol. 94 (14), e00480-e00520 (2020).
- van der Hee, B., Madsen, O., Vervoort, J., Smidt, H., Wells, J. M. Congruence of transcription programs in adult stem cell-derived jejunum organoids and original tissue during long-term culture. Front Cell Dev Biol. 8, 375 (2020).
- van der Hee, B., et al. Optimized procedures for generating an enhanced, near physiological 2D culture system from porcine intestinal organoids. Stem Cell Res. 28, 165-171 (2018).
- Mussard, E., et al. Culture of piglet intestinal 3D organoids from cryopreserved epithelial crypts and establishment of cell monolayers. J Vis Exp. (192), e64917 (2023).
- Ussing, H. H., Zerahn, K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiologica Scandinavica. 23 (2-3), 110-127 (1951).
- Dengler, F., Kraetzig, A., Gabel, G. Butyrate protects porcine colon epithelium from hypoxia-induced damage on a functional level. Nutrients. 13 (2), 305 (2021).
- Guschlbauer, M., et al. Trans-resveratrol and epsilon-viniferin decrease glucose absorption in porcine jejunum and ileum in vitro. Comp Biochem Physiol A Mol Integr Physiol. 165 (3), 313-318 (2013).
- Herrmann, J., Hermes, R., Breves, G. Transepithelial transport and intraepithelial metabolism of short-chain fatty acids (SCFA) in the porcine proximal colon are influenced by SCFA concentration and luminal pH. Comp Biochem Physiol A Mol Integr Physiol. 158 (1), 169-176 (2011).
- Klinger, S., Breves, G. Resveratrol inhibits porcine intestinal glucose and alanine transport: Potential roles of Na(+)/K(+)-ATPase activity, protein kinase A, AMP-activated protein kinase and the association of selected nutrient transport proteins with detergent resistant membranes. Nutrients. 10 (3), 302 (2018).
- Leonhard-Marek, S., Hempe, J., Schroeder, B., Breves, G. Electrophysiological characterization of chloride secretion across the jejunum and colon of pigs as affected by age and weaning. J Comp Physiol B. 179 (7), 883-896 (2009).
- Schroeder, B., et al. Preventive effects of the probiotic Escherichia coli strain Nissle 1917 on acute secretory diarrhea in a pig model of intestinal infection. Dig Dis Sci. 51 (4), 724-731 (2006).
- Hoffmann, P., et al. Intestinal organoid-based 2D monolayers mimic physiological and pathophysiological properties of the pig intestine. PLoS One. 16 (8), e0256143 (2021).
- Ferreira, S. H., Moncada, S., Vane, J. R. Indomethacin and aspirin abolish prostaglandin release from the spleen. Nat New Biol. 231 (25), 237-239 (1971).
- Dykstra, G. D., Kawasaki, M., Ambrosini, Y. M. Advancements in bovine organoid technology using small and large intestinal monolayer interfaces. J Vis Exp. (208), e67010 (2024).
- Srinivasan, B., et al. TEER measurement techniques for in vitro barrier model systems. J Lab Autom. 20 (2), 107-126 (2015).
- Hoffmann, P., et al. Caco-2/HT29-MTX co-cultured cells as a model for studying physiological properties and toxin-induced effects on intestinal cells. PLoS One. 16 (10), e0257824 (2021).
- Beduneau, A., et al. A tunable Caco-2/HT29-MTX co-culture model mimicking variable permeabilities of the human intestine obtained by an original seeding procedure. Eur J Pharm Biopharm. 87 (2), 290-298 (2014).
- Markov, A. G., Veshnyakova, A., Fromm, M., Amasheh, M., Amasheh, S. Segmental expression of claudin proteins correlates with tight junction barrier properties in rat intestine. J Comp Physiol B. 180 (4), 591-598 (2010).
- Pearce, S., et al. Marked differences in tight junction composition and macromolecular permeability among different intestinal cell types. BMC Biology. 16 (1), 19 (2018).
- Legen, I., Salobir, M., Kerc, J. Comparison of different intestinal epithelia as models for absorption enhancement studies. Int J Pharm. 291 (1-2), 183-188 (2005).
- Moeser, A. J., et al. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. Am J Physiol Gastrointest Liver Physiol. 292 (1), G173-G181 (2007).
- Moeser, A. J., Ryan, K. A., Nighot, P. K., Blikslager, A. T. Gastrointestinal dysfunction induced by early weaning is attenuated by delayed weaning and mast cell blockade in pigs. Am J Physiol Gastrointest Liver Physiol. 293 (2), G413-G421 (2007).
- Seamon, K. B., Padgett, W., Daly, J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 78 (6), 3363-3367 (1981).
- Herrmann, J., et al. Segmental diversity of electrogenic glucose transport characteristics in the small intestines of weaned pigs. Comp Biochem Physiol A Mol Integr Physiol. 163 (1), 161-169 (2012).
- Wright, E. M., Martin, M. G., Turk, E. Intestinal absorption in health and disease--sugars. Best Pract Res Clin Gastroenterol. 17 (6), 943-956 (2003).
- Crane, R. K. Na+ -dependent transport in the intestine and other animal tissues. Fed Proc. 24 (5), 1000-1006 (1965).
- Inagaki, A., Yamaguchi, S., Ishikawa, T. Amiloride-sensitive epithelial Na+ channel currents in surface cells of rat rectal colon. Am J Physiol Cell Physiol. 286 (2), C380-C390 (2004).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved