Method Article
Hybrid PET/MRI Imaging of Alzheimer's Disease Based on 18F-AV-1451
In This Article
Summary
This protocol describes the use of 18F-AV-1451 PET/MRI to reveal tau pathology and neurodegeneration, aiding neurologists in diagnosing Alzheimer's disease, assessing its severity, and gaining insights into its progression and underlying pathological mechanisms.
Abstract
Alzheimer's disease (AD) is a progressive and irreversible neurodegenerative disorder characterized by cognitive dysfunction, decline in daily living abilities, and behavioral changes, imposing a significant economic and social burden worldwide. One of the primary pathological hallmarks of AD is the accumulation of neurofibrillary tangles formed by hyperphosphorylated tau protein. Positron emission tomography/magnetic resonance imaging (PET/MRI) provides detailed structural and functional information along with specific protein distributions, making it an increasingly valuable tool for AD diagnosis and research. 18F-AV-1451 is a radiotracer specifically designed for tau protein detection in PET imaging of brain tissue. This study presents a detailed protocol for the radiosynthesis of 18F-AV-1451, patient preparation, PET/MRI image acquisition techniques, and its potential applications in AD evaluation. 18F-AV-1451 PET/MRI could aid neurologists in diagnosing AD, assessing disease severity, and gaining insights into its pathological mechanisms. In conclusion, this protocol provides a sensitive, comprehensive, and non-invasive approach for the evaluation of AD, offering valuable insights into disease progression and pathology.
Introduction
Alzheimer's disease (AD) is a progressive and irreversible neurodegenerative disorder. Patients typically experience cognitive dysfunction, a decline in daily living abilities, and behavioral changes. With the accelerating global aging population, Alzheimer's disease has become a major public health concern. Currently, more than 50 million people worldwide are living with AD. By 2050, the prevalence of dementia is expected to triple globally1. Approximately 10.8% of individuals aged 65 and older have Alzheimer's dementia. The prevalence of AD is about 5% in individuals aged 65 to 74, increasing to 33.3% in those aged 85 and older, making it one of the leading causes of death among the elderly2. Once a patient develops AD, it places a significant burden on the family3. Due to its insidious onset, patients often miss the optimal window for treatment after diagnosis. Its early detection remains a global challenge4, as the exact etiology is still not completely understood5,6. Given the complex etiology and pathways influencing AD, there is an urgent need for more accurate and early diagnostic strategies.
Conventional imaging methods such as computed tomography (CT) and magnetic resonance imaging (MRI) are used to observe atrophy or other structural changes in the brain, which may contribute to cognitive impairment. MRI, in particular, provides better soft tissue contrast than CT and offers tumor biology information without exposing patients to ionizing radiation, making it the preferred imaging modality for most neurological disorders6. MRI is a powerful imaging technique that not only provides detailed macroscopic anatomical information but also includes various functional imaging methods, such as blood oxygen level-dependent functional magnetic resonance imaging (BOLD fMRI), which can be used to observe functional brain activity6. Positron emission tomography (PET) is a non-invasive molecular imaging method that enables extensive semi-quantitative analysis of biological processes in the human brain. Hybrid PET/MRI may offer advantages over other imaging techniques for AD diagnosis7, as the use of a wide variety of PET tracers can provide additional physiological information to complement anatomical MRI images8,9.
Neurofibrillary tangles formed by the hyperphosphorylation of tau protein are one of the pathological features of AD, closely associated with the onset of neurodegeneration and the manifestation of clinical symptoms both spatially and temporally6. Tau protein is the most abundant microtubule-associated protein in vivo and is prevalent in both the peripheral and central nervous systems. The pathological progression of tau is strongly correlated with the degree of cognitive impairment and has the potential to serve as a therapeutic target for AD patients7. Non-invasive detection of tau protein deposition in specific brain regions is valuable for the early prediction and diagnosis of the disease. The use of tau radiotracers enables the visualization and localization of tau protein deposits in the brain, providing timely and accurate differential diagnosis, as well as valuable support in tracking disease progression and assessing experimental clinical treatments8,9.
Several studies have demonstrated a relationship between changes in specific proteins and functional or morphological MRI findings10,11. However, there are limited reports on combined analyses using amyloid/tau imaging and functional MRI12. The novel molecular diagnostic drug 18F-AV-1451 (7-(6-[18F]fluoropyridin-3yl)-5H-pyrido[4,3-b]indole) has been used as a radiodiagnostic agent for tau protein detection in PET-imaged brain tissue. Research on tau PET imaging is still in its developmental stage, with only a few tracers currently evaluated, including 18F-T807 (18F-AV-1451), 18F-T808 (18F-AV-680)13, 11C-PBB314, 18F-THK-511715, 18F-THK-52316, and 18F-THK-510517,18. 18F-AV-1451 was commercially developed19and has been reported for use in patients with AD20, progressive supranuclear palsy21, and dementia with Lewy bodies22. The radiosynthesis process of 18F-AV-1451 requires a complex purification step to ensure that the final product meets dosage requirements for clinical imaging studies23. With the increasing application of 18F-labeled radiotracers in PET imaging technology, there is a growing demand for the synthesis and development of new 18F-labeled radiotracers. This study presents a tau PET/MRI imaging method aimed at providing more detailed information for the diagnosis of patients with Alzheimer's disease.
Protocol
The study was approved by the local Medical Ethics Committee, and all subjects provided written informed consent prior to participation. All studies were conducted in adherence to the tenets of the Declaration of Helsinki. All subjects underwent neurological and neuropsychological evaluations by a healthcare professional within three months before and after imaging. Patients were included based on the National Institute on Aging-Alzheimer's Disease Association (NINCDS-ADRDA) criteria24 and the National Institute on Aging-Alzheimer's Association (NAA) criteria25. The details of the reagents and equipment used are listed in the Table of Materials.
1. 18F-AV-1451 synthesis
NOTE: Follow the principles of biological and radiological occupational protection, as well as the principles of medical and radioactive waste disposal, during operations.
- Synthetic details
- Synthesize 2.5 mL of [18F] fluoride using the 18O(p, n)18F nuclear reaction by employing a particle accelerator, with protons at integrated currents up to 45 µA bombarding for 40 min. Add [18F] fluoride to the automated synthesis module via the transfer pipeline, facilitated by helium gas overpressure. Operate the synthesis module in the order shown in Figure 1.
- Trap [18F] fluoride on an ion-exchange column (pre-activate with 1 mL of ethanol and 2 mL of water) while retaining the 18O water in the recovery vessel.
- Allow potassium carbonate solution from No.1 vessel (Figure 1) to flow through designated valves and ion-exchange column to exchange with [18F] fluoride, then elute into the reaction vessel. See Table 1 for solutions in the different vessels.
- Dissolve [18F] fluoride and a cryptand-based phase-transfer catalyst pre-loaded into the No.2 vessel in the reaction vessel.
- Open the designated valve. Subject the reaction mixture to vacuum distillation under nitrogen blowing at 85 °C for 8 min, followed by nitrogen blowing at 110 °C for 4 min to eliminate residual water.
- Add the precursor from the No.3 vessel into the No.12 vessel and heat to 130 °C for 10 min. The radiosynthesis of 18F-AV-1451 is shown in Figure 2.
- Cool the reaction mixture to 50 °C, then open the designated valves to restore atmospheric pressure.
- Flush the reaction mixture into the No.14 vessel using a volume of 3 mL HPLC mobile phase (25% ethanol aqueous solution, pH adjusted to 2.0) sourced from No.5 and No.6 vessels. Open the designated valves to enable the ingress of the mixture into the HPLC loop under helium pressure.
- Introduce the product into the HPLC semi-preparative column and elute with the mobile phase at a flow rate of 5 mL/min. Observe the eluent using UV (λ = 254 nm) and radioactivity counters.
- Collect the product into the round-bottom flask via the designated valve.
- Entrap and retain the product within the C18 column (activated by 5 mL of ethanol and 10 mL of water).
- Open the designated valve, wash the column with water from the No.9 vessel into the waste container, and then rinse into the product bottle with solution from No.8 and No.7 vessels.
- Pass 18F-AV-1451 through a 0.22 µm liquid filter membrane into the collection bottle in the dispensing hot cell under helium pressure.
- Quality control
NOTE: Perform quality control on three consecutive batches of the product. Ensure that the quality control of the product meets the 2020 edition of Pharmacopoeia of the People's Republic of China criteria (see Table of Materials).- Conduct visual inspection by placing the product behind lead glass and inspecting the color and clarity.
- Determine the pH value of the product using precision pH test paper.
- Use HPLC to analyze the radiochemical purity of the product8,16.
- Analyze the radioactive nuclear purity of the product using the gamma spectrometer method17.
- Measure the half-life of the product accurately according to the Pharmacopoeia of the People's Republic of China13.
- Apply a pressure of 0.34 MPa to the sterile filter membrane (step 1.1.12) to test its sealing and integrity.
- Perform sterility and endotoxin tests using bacterial culture and the horseshoe crab reagent method23.
- Test the cryptand-based phase-transfer catalyst residue using the potassium iodoplatinate method. Analyze the content of acetone, acetonitrile, and DMSO in the product using a gas chromatograph8,16.
NOTE: Ensure that no other peaks appear except 0.511 MeV and 1.022 MeV.
2. Pre-examination guidelines
NOTE: Participants were included if they had a present concern regarding a change in cognition and exhibited impairment in one or more cognitive domains while maintaining independence in functional abilities25. Eligibility required a Mini-Mental State Examination (MMSE) score between 0 and 20 and amyloid protein positivity, confirmed through an abnormal cerebrospinal fluid (CSF) Aβ42 or Aβ42/Aβ40 ratio or a positive amyloid positron emission tomography (PET) scan6,20. Exclusion criteria encompassed a diagnosis of vascular disease, depression, traumatic brain injury, psychotic disorders, or other conditions associated with cognitive decline5. Participants with contraindications to pacemakers, ferromagnetic substances, or foreign objects posing a mobility hazard, as well as those unable to undergo MRI imaging, were excluded. Additional exclusion criteria included intolerance to gadolinium contrast agents and the presence of severe renal insufficiency21.
- Instruct subjects to refrain from alcohol, caffeine, medication, or any strenuous exercise or activity for 24 h before the study. Maintain a stable physiological state (e.g., biorhythms, brain function tasks) to ensure data accuracy.
- Allow subjects to consume water and food before the examination, as these do not interfere with tau PET/MR imaging.
- Discontinue neurologic-related drugs for at least 12 h before imaging.
- Advise subjects to avoid wearing metal jewelry or clothing with metal buttons or zippers on the day of the examination.
NOTE: Ensure that the patient is accompanied by a family member, brings previous examination records, and informs medical staff about medication use.
3. Preparation for scanning
- Verify the application form to confirm general information, examination purpose, and program. Ensure that all necessary preparations have been completed. Register and file the details.
- Obtain informed consent for PET/MR imaging from the subject or a family member. Inform the subject of the purpose, procedure, risks, and benefits.
- Conduct a detailed medical history review, record the subject's height, weight, and blood concentration, and confirm the absence of contraindications to MR examination.
- Establish an intravenous line in a peripheral superficial vein or access a port catheter.
- Before administering 18F-AV-1451, verify all information again to prevent errors.
- Inject 18F-AV-1451 at 3.7-5.5 MBq/kg slowly through the intravenous access. Flush the tube with an appropriate amount of saline to minimize radioactive tracer residue. Apply compression to the injection site to prevent fluid leakage.
- Record the injection time, site, and activity.
- After completing the injection, initiate audio-visual closure. Dim the lights in the waiting
room and set the temperature to approximately 22 °C. Instruct the subject to rest in bed with eyes closed for 80 min, avoiding talking, eating, or chewing during this period.
4. PET/MRI scanning
- Instruct the subject and companion to remove all metallic objects, including mobile phones, headgear, dentures, glasses, watches, wallets, and coins, before the examination. Do not allow wheelchairs, stretchers, examination beds, oxygen cylinders, or monitoring equipment in the examination room.
- Provide earplugs to the subject and position them supine on the PET/MRI table with a head/neck coil enclosing the cervical region. Use a specialized headrest or sponge pad to stabilize the head, minimizing movement and ensuring comfort.
NOTE: Administer an appropriate dose of sedative medication, if necessary, based on neurological evaluation. - Position the subject with arms down and instruct them to use the alarm device in case of discomfort.
- Review the images to confirm that the head is centered in the scanner, aligned with the center of the coil, and positioned consistently relative to the neck.
- Use an 8-channel head and neck union coil for imaging.
- Acquire 3D brain volumetric T1-weighted sequences with high resolution and high signal-to-noise ratio (SNR) using a spoiled gradient-recalled sequence. Set the following parameters:
- Repetition time (TR) = 8.5 ms; Echo time (TE) = 3.2 ms; Inversion time (TI) = 450 ms; Flip angle = 12°; Voxel size = 1 × 1 × 1 mm³; Field of view (FOV) = 256 mm; Matrix size = 256 × 256; Slice thickness = 1 mm.
- Acquire axial PROPELLER T2-weighted sequences with the following parameters: TR = 6837 ms; TE = 132 ms; Flip angle = 142°; FOV = 240 mm; Matrix size = 416 × 416; Slice thickness = 5 mm.
NOTE: PET scanning uses a single bed position, covering the entire brain from the foramen magnum to the top of the skull.
- Acquire 20-min PET images simultaneously.
- Perform PET scanning in 3D acquisition mode.
- Use volumetric scanning for PET imaging.
- Perform MR imaging attenuation correction using a zero echo time (ZTE) pulse sequence to segment bone, air, and soft tissue.
- Acquire PET data using the time-of-flight ordered subset expectation maximization (OSEM) for image reconstruction20,23. Use the following parameters:
- Matrix size = 192 × 192; 28 subsets with 6 iterations; FOV = 350 × 350 mm; Full-width at half maximum (FWHM) = 3.0 mm.
- Calculate standardized uptake value (SUV) using the formula20,23:
SUV = (Sphere activity (Bq/ml) × Body weight (kg)) / Injected dose.
5. Image interpretation
- Visually assess all PET/MR images independently by at least two experienced nuclear medicine physicians blinded to the clinical diagnosis.
- Identify brain lobes exhibiting abnormal structural changes and protein deposition.
- Repeat the visual assessment until a final consensus is reached.
- Define a "positive scan" as an increased 18F-AV-1451 uptake in any or all cortical regions. Define a "negative scan" as the absence of 18F-AV-1451 uptake in all cortical regions.
- Define atrophy in the medial temporal lobe, particularly in the hippocampus on MRI, as neurodegeneration20,25.
Results
18F-AV-1451 synthesis results
The one-step synthesis of 18F-AV-1451 under optimized reaction conditions increased the synthesis yield to 25.7% ± 5.8%. The total reaction time was 70 min. A typical semi-preparative HPLC and UV spectrum is shown in Figure 3, where peak 2 represents the product peak.
Quality test results
For three consecutive batches, the quality control results were as follows: The solution was colorless, visually transparent, and free of obvious impurities. The pH ranged from 4.5 to 7.5. HPLC and TLC analyses showed that the chemical purity was >95% and the radiochemical purity was >95%. The primary photon energy was 0.511 MeV. The half-life met the required specifications, excluding long half-life isotopes (t1/2 > 5 days). The sterile filter membrane was pressurized to 0.34 MPa without air leakage. Endotoxin levels were <15 EU per 1 mL of the product. Bacterial culture confirmed sterility. K2.2.2 concentration was ≤50 µg/mL. Additionally, residual solvents such as acetone, acetonitrile, and DMSO met the standards of the Pharmacopoeia of the People's Republic of China (see Table of Materials).
18F-AV-1451 PET/MR results
Figure 4 presents an example of PET/MR imaging in a 69-year-old man with persistent cognitive decline (MMSE score: 19/30) with positive tau deposition. The images show significant brain atrophy, primarily characterized by widened and deepened gray matter gyri and enlarged ventricles. The AD subgroups exhibited significant 18F-AV-1451 binding with the varied regional distribution. Tau accumulation began in the entorhinal cortex and hippocampus, then spread to the temporal and parietal cortices, and eventually extended extensively throughout the cerebral cortex.
Figure 5 presents an example of PET/MR imaging in a 68-year-old man with cognitive decline (MMSE score: 24/30) with negative tau deposition. The images show normal brain parenchyma morphology bilaterally, with uniform and symmetrical 18F-AV-1451 radioactivity uptake. MRI from the same imaging session showed normal ventricular size and morphology, with no midline displacement and no abnormal signals detected in the brain parenchyma. There was nonspecific uptake in the basal ganglia and brainstem.
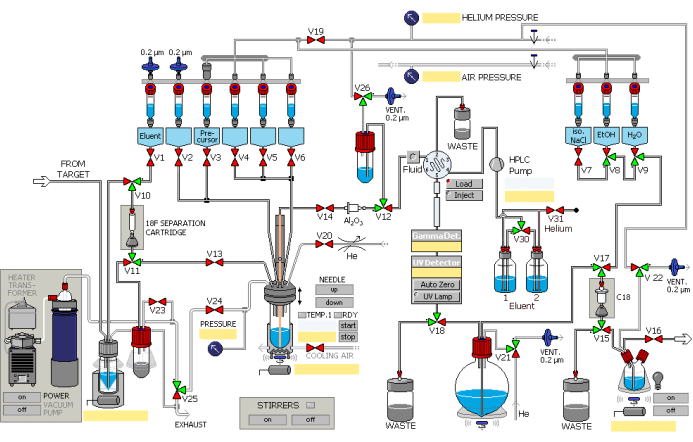
Figure 1: Schematic diagram of the radiosynthesis module. The synthesis module operates sequentially as per the designated steps. Please click here to view a larger version of this figure.

Figure 2: Radiosynthesis of 18F-AV-1451. The compound was synthesized using a one-pot method under optimized conditions. The nitro-precursor was reacted with K[18F]/K2.2.2 in DMSO at 130 °C for 10 min. Please click here to view a larger version of this figure.

Figure 3: HPLC (A) and UV (B) separation of 18F-AV-1451radioactivity. The semi-preparative HPLC and UV spectra are shown. Peak 2 represents the product peak. Please click here to view a larger version of this figure.

Figure 4: Representative imaging data of a patient with positive tau deposition. (A) 18F-AV-1451 PET images. (B) Axial T1-weighted images (T1WI). (C) Axial T2-weighted images (T2WI). (D) Axial 18F-AV-1451 PET/MR fusion images. T1WI and T2WI images show bilateral hippocampal volume reduction, ventricular dilation, widening and deepening of the sulcal fissures, and no midline structure displacement. 18F-AV-1451 PET/MR fusion images reveal a positive tau PET scan, with uptake in the medial frontal gyrus, anterior cuneus, inferior temporal gyrus, and lateral occipital cortex. Please click here to view a larger version of this figure.
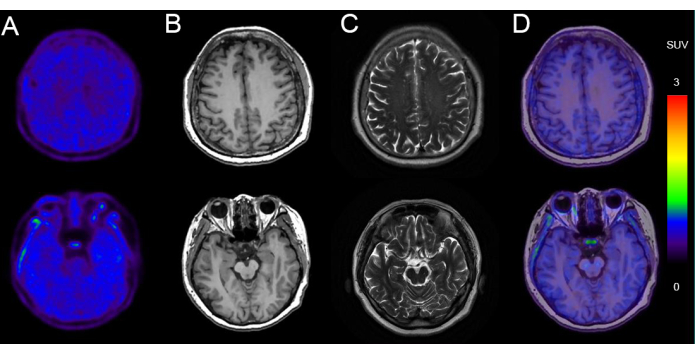
Figure 5: Representative imaging data of a patient with negative tau deposition. (A) 18F-AV-1451 PET images. (B) Axial T1-weighted images (T1WI). (C) Axial T2-weighted images (T2WI). (D) Axial 18F-AV-1451 PET/MR fusion images. T1WI and T2WI images show a normal brain MRI, with no significant temporal lobe atrophy indicative of neurodegeneration. 18F-AV-1451 PET/MR fusion images demonstrate a normal tau PET scan, with no specific cortical uptake suggestive of tauopathy. Please click here to view a larger version of this figure.
| Solvent bottle | solvent |
| No.1 | 1.5 mg K2CO3 in 0.5 ml water |
| No.2 | 1.5 mg K2.2.2. in 1 ml acetonitrile |
| No.3 | 1 mg precursor in 1.2 ml DMSO solvent |
| No.5 | 1.5 ml HPLC mobile phase |
| No.6 | 1.5 ml HPLC mobile phase |
| Round-bottom flask | 2 ml 84% NaHCO3 aqueous solution and 30 ml water |
| No.7 | 9 ml 0.9% saline |
| No.8 | 1 ml EtOH |
| No.9 | 10 ml water |
Table 1: Solutions present in different vessels of the radiosynthesis module.
Discussion
In this methodological manuscript, we have introduced an updated radiosynthesis of 18F-AV-1451 for tau PET imaging and the techniques for 18F-AV-1451 PET/MRI image acquisition, and provided the potential utility of 18F-AV-1451 PET/MR for evaluating AD. Conventional brain CT or MRI usually provides macroscopic anatomic structural assessment, which limits the assessment of disease progression and prediction of prognosis. PET is a neuroimaging tool that makes it possible to measure in vivo molecular processes.PET radioligands can bind targets, including receptors, transporter proteins, or enzymes26. The technology has been used in the diagnosis of neurological disorders, the development of programs, and the assessment of disease progression. PET/MRI is a novel hybrid functional brain imaging system that combines positron emission tomography (PET) and MRI. Because of the wide variety of PET tracers, PET/MR can provide additional physiologic information beyond anatomical MRI images.Simultaneous acquisition of PET and MRI data will help to take advantage of the inherent advantages of both technologies and eliminate the potential delay of two separate inspections, while also increasing efficiency and convenience for the patient. PET/MRI removes the additional radiation required for attenuation correction and anatomical correlation in the CT portion of a PET/CT, which can be a great advantage for special patients such as pregnant women, young children, and patients who need multiple PET imaging exams.
The National Institute on Aging and Alzheimer's Association (NIA-AA) proposed a diagnostic framework (A/T/N framework) in 2011 based on biological markers27, including β-amyloid (Aβ) deposition (A), pathologic tau (T), and neurodegeneration (N)28,29. Aβ is the component of senile plaques, whose markers include measurements of reduced levels of Aβ42 in cerebrospinal fluid (CSF), and Aβ deposition demonstrated by PET. Pathologic tau is the main component of neurofibrillary tangles fibrosis, whose markers include increased total tau or phosphorylated tau (p-tau) in CSF30,31,32,33. Measures of neurodegeneration include brain atrophy rates34,35 and hippocampal volume or medial temporal lobe atrophy36,37detected by MRI, and hypometabolism or hypoperfusion obtained with PET and single photon emission computed tomography (SPECT) imaging38,39. PET/MRI may offer advantages over other imaging methods in the diagnosis of AD in providing simultaneous pathological protein deposition and atrophy in the hippocampal region in a single scan.
Recently, new PET tracers were made available for in vivo non-invasive detection of tau, the accumulation of which is a defining characteristic of AD pathology40. 18F-AV1451 exhibits potent binding and selectivity for tau in brain tissue of AD patients41, with relatively slower kinetics. We used 18F-AV1451 to examine the distribution of tau proteins in the brains of AD patients. 18F-AV-1451 has been synthesized in multiple methods. This study automated the synthesis of 18F-AV-1451 on the TRACERlab FXFN synthesizer using a one-pot method with optimized conditions. The precursor dosage changed to 1 mg42. Simultaneously, HPLC separation conditions were adjusted to a 25% EtOH aqueous solution with pH adjusted to 2.0 for online deprotection of BOC. Solvent conversion and re-purification on a C18 column were performed. The yield of the synthesis was increased from 20.5% ± 6.1% to 25.7% ±5.8% by applying the improved synthesis method.The method improved the product yield. Various quality control tests performed after synthesis showed that the product met the quality control standards.
The results showed that AD patients had obvious brain atrophy, with significant binding of 18F-AV-1451.The accumulation of tau begins in the entorhinal cortex and hippocampus, then spreads to the temporal and parietal cortices, and finally remains extensively throughout the cerebral cortex. Tau PET is a promising tool, and several studies have already assessed the diagnostic value of tau-PET. Studies showed that tau-PET, the binding patterns, corresponds well with the Braak histopathologic descriptions43,44, which is a staging framework that corresponds well with the clinical status across the AD spectrum45,46. Tau load and topography correlate better with clinical symptoms than amyloid load47.
The NIA-AA classified "A" positivity as "Alzheimer's Continuum" in 201129. In 2018, the framework for biomarker-based AD research was established and refined, stating that patients in this stage have the pathologic changes of AD27. In the revised criteria for the staging of AD, published in 2024, it is noted that A+T- are Initial-stage48. In some cases, it may be many years before they appear clinical symptoms. Individuals who are positive for biomarkers in both the "A" and "T" categories are classified as having AD. Previous studies showed that tau-negative individuals were less likely to present with AD features and that most patients do not progress to dementia during at least 5 years of follow-up49. Patients with tau-positive MCI may evolve into AD. If the patient has a broad uptake of T and is positive for N, that means that they are in an advanced stage of the disease. Therefore, detection of tau pathology and neurodegeneration is essential for accurate patient management. Previous studies have indicated that individuals with tau negativity were less likely to exhibit clinical features associated with Alzheimer's disease, and most did not develop dementia over a follow-up period of at least five years49. The early in vivo diagnosis of mild cognitive impairment with tau positivity, which may progress to Alzheimer's disease, is essential for precise patient management.
Previously, information obtained with PET and MRI required parallel analysis of sequentially acquired data followed by post hoc fusion to provide good spatial registration. However, a subject's mental state and physiologic or cognitive conditions may change between examinations. Hybrid PET/MR displays different physiologic and metabolic information about the disease process by temporally and spatially matching datasets.
Disclosures
The authors have no conflict of interest to declare.
Acknowledgements
This work was supported by grants from the Key Research and Development Plan of Liaoning Province (2019JH/10300010) and the Applied Basic Research Program of Liaoning Province (2022JH2/101500011).
Materials
| Name | Company | Catalog Number | Comments |
| 18O-rich water | Taiyo Nippon Sanso,Japan | 24-0091 | |
| 2020 edition of Pharmacopoeia of the People's Republic of China | NA | https://english.nmpa.gov.cn/2020-07/03/c_538689.htm | |
| Acetonitrile | ABX,Germany | TF-A1-231207002 | |
| air filter membrane (Millex-25) | Merck,Germany | SLFGN25VS | |
| Boc-protected-precursor | Huayi,Jiangsu,China | NPPI-95-0001A | |
| C18 column | Waters,USA | 186004770 | |
| Cyclotron | GE,USA | MINITRACE | |
| Dimethyl sulfoxide (DMSO) | Bailing Wei Technology,Beijing,China | 984549 | |
| EtOH | ABX,Germany | 10009216 | |
| EtOH for isolation | Sinopharm Chemical Reagent,Shanghai,China | 400212682 | |
| Gas chromatography | Tianmei,Shanghai,China | GC-7900 | |
| HPLC | SYKNM,Germany | S-1122 | |
| Hydrochloric acid | Sinopharm Chemical Reagent,Shanghai,China | 10011018 | |
| K2CO3 Solution | ABX,Germany | TF-K1-230724001 | |
| Kryptofix[2.2.2](K222) | ABX,Germany | 800 | |
| liquid filter membrane (Millex-GV) | Merck,Germany | SLGVR33RB | |
| Oasis HLB solid-phase extraction (SPE) column | Waters,USA | 186003908 | |
| PET/MR | GE,USA | Signa | |
| QMA column | Waters,USA | 186002350 | |
| Radionuclide activity | Capintec,USA | CRC-25R |
References
- Scheltens, P., et al. Alzheimer's disease. Lancet. 397 (10284), 1577-1590 (2021).
- Rajan, K. B., et al. Population estimate of people with clinical AD and mild cognitive impairment in the United States (2020-2060). Alzheimers Dement. 17 (12), 1966-1975 (2021).
- U.S. Burden of Disease Collaborators. The state of U.S. health, 1990-2016: Burden of diseases, injuries, and risk factors among U.S. states. JAMA. 319 (14), 1444-1472 (2018).
- Bhatt, J., et al. . The World Alzheimer Report 2019: Attitudes to dementia. , (2019).
- Rostagno, A. A. Pathogenesis of Alzheimer's disease. Int J Mol Sci. 24 (1), 107 (2022).
- Ossenkoppele, R., van der Kant, R., Hansson, O. Tau biomarkers in Alzheimer's disease: Towards implementation in clinical practice and trials. Lancet Neurol. 21 (8), 726-734 (2022).
- Abbott, A. Conquering Alzheimer's: A look at the therapies of the future. Nature. 616 (7955), 26-28 (2023).
- Politis, M., Piccini, P. Positron emission tomography imaging in neurological disorders. J Neurol. 259 (9), 1769-1780 (2012).
- Okamura, N., et al. Tau PET imaging in Alzheimer's disease. Curr Neurol Neurosci Rep. 14 (11), 500 (2014).
- Holland, N., et al. Differential synaptic loss in β-amyloid positive versus β-amyloid negative corticobasal syndrome. Mov Disord. 39 (7), 1166-1178 (2024).
- Nicastro, N., et al. 18F-AV1451 PET imaging and multimodal MRI changes in progressive supranuclear palsy. J Neurol. 267 (2), 341-349 (2020).
- Cousins, O., et al. Microglial activation, tau and amyloid deposition in TREM2 p.R47H carriers and mild cognitive impairment patients: A multi-modal/multi-tracer PET/MRI imaging study with influenza vaccine immune challenge. J Neuroinflammation. 20 (1), 272 (2023).
- Chien, D., et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F18]-T808. J Alzheimers Dis. 38 (1), 171-184 (2014).
- Maruyama, M., et al. Imaging of tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron. 79 (6), 1094-1108 (2013).
- Li, Y., et al. Cortical laminar binding of PET amyloid and tau tracers in Alzheimer disease. J Nucl Med. 56 (2), 270-273 (2015).
- Fodero-Tavoletti, M. T., et al. 18F-THK523: A novel in vivo tau imaging ligand for Alzheimer's disease. Brain. 134 (4), 1089-1100 (2011).
- Okamura, N., et al. Non-invasive assessment of Alzheimer's disease neurofibrillary pathology using 18F-THK5105 PET. Brain. 137 (Pt 6), 1762-1771 (2014).
- Tago, T., et al. Preclinical evaluation of [(18)F]THK-5105 enantiomers: Effects of chirality on its effectiveness as a tau imaging radiotracer. Mol Imaging Biol. 18 (2), 258-266 (2016).
- Xia, C. F., et al. [18F]T807, a novel tau positron emission tomography imaging agent for Alzheimer's disease. Alzheimers Dement. 9, 666-676 (2013).
- Fleisher, A. S., et al. Positron emission tomography imaging with [18F]flortaucipir and postmortem assessment of Alzheimer disease neuropathologic changes. JAMA Neurol. 77 (7), 829-839 (2020).
- Whitwell, J., et al. [18F]AV-1451 tau positron emission tomography in progressive supranuclear palsy. Mov Disord. 32 (1), 124-133 (2017).
- Kantarci, K., et al. AV-1451 Tau and β-amyloid positron emission tomography imaging in dementia with Lewy bodies. Ann Neurol. 81 (1), 58-67 (2017).
- Mossine, A. V., et al. An updated radiosynthesis of [18F]AV1451 for tau PET imaging. EJNMMI Radiopharm Chem. 2 (1), 7 (2017).
- McKhann, G., et al. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 34 (7), 939-944 (1984).
- McKhann, G. M., et al. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 7 (3), 263-269 (2011).
- Chandra, A., et al. Alzheimer's Disease Neuroimaging Initiative. Applications of amyloid, Tau, and neuroinflammation PET imaging to Alzheimer's disease and mild cognitive impairment. Hum Brain Mapp. 40 (18), 5424-5442 (2019).
- Jack, C. R., et al. NIA-AA research framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement. 14 (4), 535-562 (2018).
- McKhann, G. M., et al. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging and the Alzheimer's Association Workgroup. Alzheimers Dement. 7, 263-269 (2011).
- Albert, M. S., et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: Recommendations from the National Institute on Aging and Alzheimer's Association Workgroup. Alzheimers Dement. 7, 270-279 (2011).
- Simrén, J., Elmgren, A., Blennow, K., Zetterberg, H. Fluid biomarkers in Alzheimer's disease. Adv Clin Chem. 112, 249-281 (2023).
- Molinuevo, J. L., et al. Current state of Alzheimer's fluid biomarkers. Acta Neuropathol. 136, 821-853 (2018).
- Alcolea, D., et al. Amyloid precursor protein metabolism and inflammation markers in preclinical Alzheimer disease. Neurology. 85, 626-633 (2015).
- Ia, J., et al. Biomarker changes during 20 years preceding Alzheimer's disease. N Engl J Med. 390 (8), 712-722 (2024).
- Leung, K. K., et al. Cerebral atrophy in mild cognitive impairment and Alzheimer disease: Rates and acceleration. Neurology. 80 (7), 648-654 (2013).
- Contador, J., et al. Longitudinal brain atrophy and CSF biomarkers in early-onset Alzheimer's disease. Neuroimage Clin. 32, 102804 (2021).
- Menéndez-González, M., de Celis Alonso, B., Salas-Pacheco, J., Arias-Carrión, O. Structural neuroimaging of the medial temporal lobe in Alzheimer's disease clinical trials. J Alzheimers Dis. 48 (3), 581-589 (2015).
- van Oostveen, W. M., de Lange, E. C. M. Imaging techniques in Alzheimer's disease: A review of applications in early diagnosis and longitudinal monitoring. Int J Mol Sci. 22 (4), 2110 (2021).
- Reiman, E. M., Jagust, W. J. Brain imaging in the study of Alzheimer's disease. Neuroimage. 61 (2), 505-516 (2012).
- Herholz, K. Perfusion SPECT and FDG-PET. Int Psychogeriatr. 23 (Suppl 2), S25-S31 (2011).
- Lamontagne-Kam, D., et al. Implication of tau propagation on neurodegeneration in Alzheimer's disease. Front Neurosci. 17, 1219299 (2023).
- Gomar, J. J., et al. Increased retention of tau PET ligand [18F]-AV1451 in Alzheimer's disease psychosis. Transl Psychiatry. 12 (1), 82 (2022).
- Shoup, T. M., et al. A concise radiosynthesis of the tau radiopharmaceutical [(18)F]T807. J Labelled Compd Radiopharm. 56 (14), 736-740 (2013).
- Schwarz, A. J., et al. Regional profiles of the candidate tau PET ligand 18F-AV-1451 recapitulate key features of Braak histopathological stages. Brain. 139, 1539-1550 (2016).
- Pascoal, T. A., et al. Longitudinal 18F-MK-6240 tau tangles accumulation follows Braak stages. Brain. 144, 3517-3528 (2021).
- Braak, H., Braak, E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 82, 239-259 (1991).
- Braak, H., et al. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 112, 389-404 (2006).
- Ossenkoppele, R., et al. Associations between Tau, Aβ, and cortical thickness with cognition in Alzheimer disease. Neurology. 92, e601-e612 (2019).
- Jack, C. R., et al. Revised criteria for diagnosis and staging of Alzheimer's disease: Alzheimer's Association Workgroup. Alzheimers Dement. 20 (8), 5143-5169 (2024).
- Josephs, K. A., Weigand, S. D., Whitwell, J. L. Characterizing amyloid-positive individuals with normal tau PET levels after 5 years. Neurology. 98, e2282-e2292 (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved