Method Article
Robotic Vagus-Sparing Total Gastrectomy for CDH1 Gene Mutation Treatment
In This Article
Summary
This article focuses on robotic vagus-sparing total gastrectomy. The techniques and pitfalls of vagus preservation, sutured esophagojejunostomy, jejunal pouch formation, and Roux-en-Y reconstruction with a staple-stapled jejunojejunostomy are discussed.
Abstract
Hereditary diffuse gastric cancer (HDGC) caused by the CDH1 gene mutation is an inherited cancer syndrome that increases the risk of diffuse gastric cancer and is nearly impossible to detect by screening gastroscopy. The recommended preventative treatment is a total gastrectomy. Robotic surgery facilitates the use of minimally invasive surgical (MIS) techniques for anastomoses and posterior vagus preservation to potentially reduce adverse functional outcomes. An asymptomatic 24 year old male with the CDH1 gene mutation proven by genetic testing and a family history of a brother having a total gastrectomy for HDGC was treated with this technique. This video case report demonstrates the techniques and pitfalls of robotic surgery in terms of the patient positioning and port placement, posterior vagus-preserving dissection, sutured esophagojejunostomy, jejunal pouch formation, and Roux-en-Y reconstruction with a staple-stapled jejunojejunostomy. While these techniques are demonstrated in the case of prophylactic gastrectomy, many of them can be applied to other benign and bariatric foregut and general surgery types.Robotic surgery can facilitate the foregut MIS technique, as described in this case of a vagus-sparing total gastrectomy.
Introduction
Hereditary diffuse gastric cancer (HDGC) is characterized by a genetic mutation in the E-cadherin (CDH1) tumor suppressor gene, which has an autosomal dominant pattern of inheritance1. This inherited cancer syndrome increases the risk of diffuse gastric cancer and lobular breast cancer (LBC). Current guidelines recommend testing for CDH1 mutations in patients with familial clusters of HDGC and LBC, particularly in those with early onset (before 40 years of age)2. According to the largest reported series of CDH1 mutation carriers, the cumulative lifetime incidence of gastric cancer is 70% (95% CI, 59%-80%) for males and 56% (95% CI, 44%-69%) for females with this mutation3. However, recent studies have estimated gastric cancer penetrance with this mutation to be in the range of 37%-42% for males and 25%-33% for females1.
Endoscopic surveillance with biopsies is the recommended surveillance type for those choosing to delay prophylactic gastrectomy; however, it is nearly impossible to detect early gastric cancer in this cohort using screening gastroscopy2. Extensive white-light endoscopic examination is followed by a minimum of 30 non-targeted gastric biopsies from five separate areas of the stomach. However, this surveillance method has a high false-negative rate and only detects 20%-63% of occult signet ring cell foci4,5.
Prophylactic total gastrectomy (PTG) is the recommended preventative treatment for any pathogenic or likely pathogenic CDH1 variant carrier starting at the age of 20 years, but it is not recommended beyond 70 years of age1,2. Perigastric lymph node metastasis is an uncommon finding in asymptomatic patients, who typically have T1a or in situ signet ring cells. Perioperative morbidity is generally low, and patient satisfaction is high6. Though the overall quality of life following surgery is high, a truncal vagotomy is generally performed in a total gastrectomy7,8. The resection of the vagus nerve above the level of the celiac and hepatic branches leads to parasympathetic denervation of the hepatobiliary tree and the small and large intestine. Post-vagotomy diarrhea and dumping syndrome are well-recognized long-term complications following PTG8.
Robotic surgery provides the surgeon with a 3-dimensional, 10x magnified view of the surgical field and offers a high degree of freedom with articulating surgical instruments9. The aim of preforming this technique is to potentially reduce adverse functional outcomes through posterior vagus preservation, which is facilitated by minimally invasive surgical (MIS) techniques.
Protocol
The patient provided informed consent for the publication of de-identified information, images, and video documentation. Associate Professor Dr. Michael Talbot (co-author) is an upper gastrointestinal surgeon accredited to perform gastrectomy at his institution. Due to the negligible risk of this case report and protocol, it was exempt from an ethics review as per the local institutional review board guidelines. Ethics application for case reports are exempted as per the local institutional review board guidelines.
1. Patient positioning
- Place the patient in the reverse Trendelenburg position after general anesthesia has been given.
- Secure the patient in position with a foot plate, a body strap placed above the knees, and ankle straps. Abduct (<90°) the arms, and crepe bandage them to the arm boards. Place a Nathanson liver retractor bracket on the operating table, cranial to the left arm board.
2. Port placement (Figure 1)
- Make a 12 mm skin incision via the surgeon's preferred approach in the right midclavicular position at or below the level of the umbilicus. Perform an optical entry with an 8 mm, 0° laparoscope scope and with a 12 mm port through this incision. Note, the camera port should be 10-20 cm from the target anatomy.
- Exchange the port for a 12 mm robotic (stapling) port with a 12-8 mm reducer, and swap the laparoscope with an 8 mm, 30° laparoscope. Establish 15 mmHg CO2 pneumoperitoneum with 45 mmHg flow.
- Make three additional 8 mm skin incisions in the transumbilical plane, and place three 8 mm robotic ports. Place one of the robotic ports at the umbilicus and the other two robotic ports lateral to it on the left side. Ensure there is a >8 cm distance between the ports and a 10-20 cm distance between the ports and the target anatomy.
- Place another 12 mm assistant port (port A) in the upper-right quadrant. Insert a Nathanson retractor into the epigastrium through a 5 mm incision, and retract the left lobe of the liver.
- Place the robotic patient cart over the patient from the patient's right side, taking care to avoid collision with the liver retractor. Dock the camera arm first using the laser-guided port placement. Perform an incisura targeting the stomach with an 8 mm, 30° robotic scope using port 3.
- Dock and burp the remaining robotics ports to the instrument arms, and then insert a force bipolar 8 mm instrument (port 1), a vessel sealer extend (port 2), and a tip-up fenestrated grasper (pot 4). Set the monopolar and bipolar auto-cut and forced coagulation at effect 3, and check the system setup.

Figure 1: Robotic port placement. The Nathanson retractor handle is seen in the epigastrium. There is a 12 mm assistant port in the upper-right quadrant. The remaining ports from the left to the right of the image are the 12 mm robotic port with reducer, the 8 mm camera port, and two additional 8 mm robotic ports. Please click here to view a larger version of this figure.
3. Vagus-preserving dissection
- Mobilize the greater curve of the stomach through the gastrocolic ligament from the antrum to the cardia and divide into five to seven short gastric vessels lateral to the arcade vessel sealer extend to include the N1 nodes. Then, mobilize the left side of the esophageal hiatus.
- Enter the phrenoesophageal ligament and mobilize the left side of the distal esophagus. Perform distal dissection with the vessel sealer and continue down to the mobilization of the first part of the duodenum. Follow the supraduodenal window through the pars flaccida to complete the right-sided esophageal mobilization, with the plane of dissection abutting the esophagus.
- Transect the first part of the duodenum with the robotic stapler using a 60 mm x 4.3 mm green reload with a buttress reinforcement. Divide the left gastric vessels close to the wall of the stomach using the vessel sealer. This is to ensure that the posterior vagus is left undivided in the mesentery around the left gastric pedicle.
- Retract the esophagus and stomach in the superolateral direction and identify the posterior vagus nerve. Sweep the nerve posteriorly and preserve it.
4. Suture of the esophagojejunostomy with a jejunal pouch
- Phrenoesophageal ligament reconstruction
- Perform the phrenoesophageal ligament reconstruction in a continuous anticlockwise direction from the 5 o'clock to 9 o'clock position using a 2-0 nonabsorbable barbed suture on a 26 mm 1/2 circle taper point needle. Transect the intraabdominal esophagus with the robotic stapler using a 60 mm x 4.3 mm green reload. Move the detached stomach to the upper-right quadrant.
- Esophagojejunal anastomosis
- Raise the greater omentum cranially to identify the duodenojejunal flexure and bring a jejunal loop of 50 cm (creating a 40-60 cm biliopancreatic limb after the jejunal pouch formation) to the esophagus.
- Suture a 23 cm long 3-0 absorbable barbed suture on a 26 mm 1/2 circle taper point needle as a 3 o'clock lateral esophagojejunal pexy. Retract the uncut suture laterally. Create an esophagostomy anterior to the staple line using a monopolar diathermy hook with an 18Fr nasogastric tube guide. Create a jejunostomy of an equal or slightly smaller size as done for esophagostomy.
- Suture the posterior wall of the esophagojejunostomy with the barbed suture in a continuous, clockwise fashion from the 3 o'clock to 9 o'clock position, incorporating the staple line. Suture the anterior portion using another of the same suture to complete the full-thickness single-layered suture for the esophagojejunal anastomosis. Place a few medial sutures beyond the anastomosis as a medial pexy.
- Jejunal pouch
- Transect the proximal jejunal loop at 10 cm from the anastomosis with a robotic stapler using a 60 mm x 2.5 mm white reload. Create antimesenteric enterostomies 10 cm proximal and distal to the esophagojejunal anastomosis.
- Perform two firings of the robotic stapler using 60 mm x 2.5 mm white reloads to create the jejunal pouch. Ensure the second firing does not go all the way to meet the esophagojejunal anastomosis in order to preserve the blood supply to the anastomoses. Close the jejunal enterotomy in a continuous fashion with a 3-0 absorbable barbed suture.
5. Stapled jejunojejunostomy
- Measure a 70 cm alimentary limb and place a 2-0 silk interrupted suture at this point and the distal biliopancreatic limb for both marking and retraction.
- Form the jejunojejunostomy using a staple-only technique, as described in the triple-stapling technique for laparoscopic gastric bypass10,11. Use a 60 mm x 2.5 mm white reload to form the anastomosis and close the enterotomy.
- Close both the jejunal and retro-Roux mesenteric defects using 2-0 nonabsorbable barbed sutures to reduce the risk of complications due to internal herniation.
6. Specimen extraction and closure
- Remove the instruments, Nathanson retractor, and ports under robotic vision. Undock the robotic patient cart from the patient.
- Create a 5 cm mini-laparotomy through the extension of the umbilical port. Deliver the specimen through a small O-ring wound protector retractor. Close the laparotomy in the usual fashion using muscle sparing sutures with continuous fascial closure and with a 1:4 suture to wound length ratio.
Results
The total operative time was 2 h 50 min, and the patient had an unremarkable postoperative course. The patient was placed on a free fluids diet on day 1 post surgery and discharged from the hospital on day 4. At the 1 month and 3-month follow-up, the patient was well and reported no diarrhea or dumping symptoms.
The specimen was sent for pathological examination and demonstrated five areas of superficial invasion of the lamina propria by signet ring cell adenocarcinoma. These areas were microscopic and confined to the lamina propria with no invasion beyond the muscularis mucosae. They were present in the distal third of the stomach, the middle portion of the stomach, and the upper third of the stomach in the cardia. These areas were in keeping with early gastric carcinoma signet ring cell carcinoma, pT1a. Eight lymph nodes were retrieved, which were unremarkable. The surgical margins were clear. No Helicobacter pylori was identified (See Figure 2).
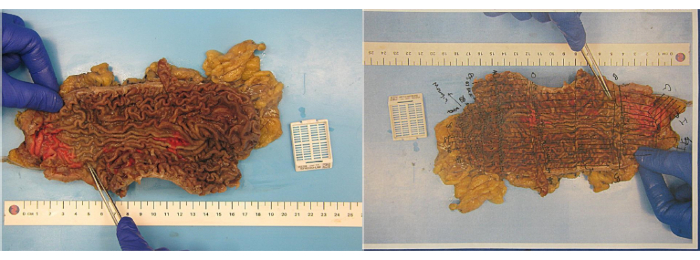
Figure 2: Macroscopic view of the total gastrectomy specimen. The mucosal surface is mildly oedematous but otherwise unremarkable. No polypoid structure or discrete lesions are identified. Please click here to view a larger version of this figure.
Discussion
An asymptomatic 24 year old male with the CDH1 gene mutation proven by genetic testing and a family history of a brother having a total gastrectomy for HDGC was selected. The preoperative endoscopy was unremarkable. The case is used as a platform to discuss the techniques and the pitfalls of key aspects of it. This includes the patient positioning and port placement, posterior vagus-preserving dissection, sutured esophagojejunostomy, jejunal pouch formation, and Roux-en-Y reconstruction with a triple stapled jejunojejunostomy, as described in the protocol.
The rate of pathological lymph node metastases in asymptomatic patients with PTG for the CDH1 mutation is extremely low12. Considering the vagus preservation and the prophylactic nature of this gastrectomy, a limited lymph node dissection was undertaken. All the resected lymph nodes were negative (0 of 8), consistent with the pathology and technique. Evidently, a more extensive lymph node dissection would be employed in patients with macroscopic disease and symptoms.
A barbed suture is preferred in our center for the even distribution of tension and the ease of handling. An absorbable monofilament suture without barbs can be used as an alternative. The reconstruction of the phrenoesophageal ligament serves as a pexy of the distal esophagus to facilitate the anastomosis and minimize retraction into the mediastinum. This reconstruction and anterior cruroplasty may also reduce the chance of future hiatal herniation.
The jejunojejunostomy is formed using a staple-only technique, as described in the triple-stapling technique for laparoscopic gastric bypass10,11. This technique is employed in this protocol to form a consistent anastomosis length with a low risk of stricture and due to our familiarity with the technique, as it is routinely used in our laparoscopic gastric bypass procedures. A hybrid suture-handsewn or totally sutured technique could be employed as an alternative.
This technique of posterior vagus-sparing total gastrectomy may potentially reduce the adverse functional outcomes, particularly diarrhea and dumping, associated with truncal vagotomy. Anterior vagal sparing is also technically possible, but this requires a full perigastric dissection of the lesser curve with no opportunity for lymph node sampling. The preservation of the anterior vagus is unlikely to offer any advantage to intestinal function post-operatively. Robotic surgery facilitates an MIS approach through the use of articulating instruments and a magnified 3-dimesional view, and this approach is advantageous when compared to traditional open and laparoscopic techniques.
The limitations of this technique relate to the use of the robotic platform. The operator and surgical assistant should be experienced with this surgical platform and performing upper gastrointestinal resection surgery.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors acknowledge the Upper Gastrointestinal and Metabolic Research Foundation for funding the journal publication fees. The authors also acknowledge the patient in this case for their consent to the publication of their de-identified information and images.
Materials
| Name | Company | Catalog Number | Comments |
| Laparoscopic instruments | |||
| 5 mm optical entry port | Applied Medical | CFF03 | Kii Fios First entry access system. |
| Laparoscopic 5mm 0° camera | Olympus | ENDOEYE HD II | 5 mm, 0° |
| Laparoscopic needle holder | KARL STORZ | Laparoscopic needle holder | |
| Nasogastric tube | Cardinal Health | 8888264960E | 16Fr |
| Nathanson liver retractor | COOK Medical | NLRS-1001/ NLRS-1002 | Large/ Extra-large |
| Robotic instruments | |||
| 12 mm port | Intuitive Surgical | 470375 | |
| 8 mm port | Intuitive Surgical | 470380 | |
| 8 mm reducer | Intuitive Surgical | 470381 | |
| Da Vinci Xi/X Endoscope with Camera, 8 mm, 0° | Intuitive Surgical | 470026 | |
| Da Vinci Xi/X Endoscope with Camera, 8 mm, 30° | Intuitive Surgical | 470027 | |
| Force Bipolar 8 mm | Intuitive Surgical | 470405 | |
| Mega SutureCut Needle Driver | Intuitive Surgical | 470309 | |
| Monopolar hook diathermy | Intuitive Surgical | 470183 | |
| SureForm 60mm stapler | Intuitive Surgical | 480460 | |
| Tip-up fenetrated grasper 8 mm | Intuitive Surgical | 470347 | |
| Vessel Sealer Extend 8 mm | Intuitive Surgical | 480422 | |
| Stapler reloads | |||
| Seamguard buttress 60 mm | GORE | 1BSGXI60GB/12BSGXI60GB | |
| SureForm 60 mm green reload | Intuitive Surgical | 48360G | |
| SureForm 60 mm white reload | Intuitive Surgical | 48360W | |
| Sutures | |||
| 2-0 nonabsorbable barbed suture | Medtronic | VLOCN0644 | 23 cm V-Loc on a 26 mm ½ circle taper point needle |
| 3-0 absorbable barbed suture | Medtronic/ Ethicon (J&J) | VLOCM0644 | 23 cm V-Loc on a 26 mm ½ circle taper point needle (preferred), STRATAFIX (alternate). Catalogue number |
| 2-0 monocryl suture | Ethicon (J&J) | JJW3463 | Cut to 15 cm, taper point needle |
| 2-0 silk suture | Ethicon (J&J) | JJ423H | Cut to 15 cm, taper point needle |
| 1 PDS suture | Ethicon (J&J) | JJ75414 | Fascial closure |
| 3-0 monocryl suture | Ethicon (J&J) | JJY227H | Skin closure |
| Topical Skin Adhesive | Ethicon (J&J) | JJ79025 | Skin closure/ wound dressing |
| Specimen extraction | |||
| Alexis O-ring wound retractor | Applied Medical | C8402 | Medium. For specimen extraction |
| Handheld diathermy | Covidien/ Valleylab | VLE2515 | For specimen extraction |
References
- Gamble, L. A., Heller, T., Davis, J. L. Hereditary diffuse gastric cancer syndrome and the role of CDH1: A review. JAMA Surgery. 156 (4), 387-392 (2021).
- Shenoy, S. CDH1 (E-cadherin) mutation and gastric cancer: Genetics, molecular mechanisms and guidelines for management. Cancer Management and Research. 11, 10477-10486 (2019).
- Hansford, S., et al. Hereditary diffuse gastric cancer syndrome: CDH1 mutations and beyond. JAMA Oncology. 1 (1), 23-32 (2015).
- Friedman, M., et al. Surveillance endoscopy in the management of hereditary diffuse gastric cancer syndrome. Clincal Gastroenterology and Hepatology. 19 (1), 189-191 (2021).
- van der Post, R. S., et al. Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. Journal of Medical Genetics. 52 (6), 361-374 (2015).
- Kaurah, P., et al. Hereditary diffuse gastric cancer: Cancer risk and the personal cost of preventive surgery. Familal Cancer. 18 (4), 429-438 (2019).
- Hejazi, R. A., Patil, H., McCallum, R. W. Dumping syndrome: Establishing criteria for diagnosis and identifying new etiolo gies. Digestive Diseases and Sciences. 55 (1), 117-123 (2010).
- Johnston, D. Operative mortality and postoperative morbidity of highly selective vagotomy. British Medical Journal. 4 (5996), 545-547 (1975).
- Nakauchi, M., et al. Robotic surgery for the upper gastrointestinal tract: Current status and future perspectives. Asian Journal of Endoscopic Surgery. 10 (4), 354-363 (2017).
- Frantzides, C. T., et al. Laparoscopic Roux-en-Y gastric bypass utilizing the triple stapling technique. Journal of the Society of Laparoendoscopic Surgeons. 10 (2), 176-179 (2006).
- Madan, A. K., Frantzides, C. T. Triple-stapling technique for jejunojejunostomy in laparoscopic gastric bypass. Archives of Surgery. 138 (9), 1029-1032 (2003).
- Forrester, J. D., et al. Surgery for hereditary diffuse gastric cancer: Long-term outcomes. Cancers. 14 (3), 728 (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved