Method Article
Intubation, Central Venous Catheter, and Arterial Line Placement in Swine for Translational Research in Abdominal Transplantation Surgery
In This Article
Summary
To obtain the best possible results, surgeons performing translational research experiments should be proficient at intubation and vascular line placement. This paper describes the techniques used by the Toronto Organ Preservation Laboratory to perform these procedures.
Abstract
Translational surgical research models in swine are crucial for developing safe preclinical protocols. However, the success of the experimental surgeries does not solely rely on the research team's surgical skills; perioperative care and management procedures, like intubation, central venous line, and arterial line placement, are necessary and of the utmost importance for favorable experiment results. As it is uncommon for research teams to have anesthesiologists or any other staff other than the surgical team, the surgical team involved in translational research must acquire and/or develop the skills to perform the perioperative care. The purpose of this paper is to show the techniques of intubation, central venous catheter, and arterial line placement used and perfected at the Toronto Organ Preservation Laboratory over the last 10 years, to be used as a reference for future researchers joining either this team or any other lab performing translational research protocols in swine and/or abdominal transplantation.
Introduction
Swine experimental models are often used in studying human diseases because of their similar anatomical and physiological properties. These models are crucial in the development of safe pre-clinical protocols, but they are also subject to legal and ethical restrictions1. The use of swine for research must be done under the best possible conditions to avoid unnecessary loss of animals and suffering due to anesthesia complications unrelated to the research project.
Pre-operative techniques and skills such as intubation, central venous line placement, and arterial line placement are essential for achieving successful and reproducible results.
Every animal undergoing general anesthesia for a surgical procedure must be intubated to maintain an open airway, allowing assisted ventilation and avoiding broncho aspiration2. The most common positions for intubation in swine are dorsal, lateral, and sternal recumbency3,4. Sternal recumbency tends to be easier for personnel trained in human intubation3, which is the case at this research facility.
Good vascular access is essential for the administration of fluids, medications, and sampling during and after surgery. The use of vasopressors is common during abdominal transplant surgeries due to the hemodynamic instability resulting from the ischemia-reperfusion injury. Infusing vasopressors through a peripheral line can cause local tissue injury due to the vasoconstrictive effects5. A central venous line placement allows for infusing large amounts of fluids and vasoppresors. We prefer a guidewire-assisted percutaneous technique for central line placement since it minimizes damage to the soft tissue and vessels6.
Hemodynamic stability of the animal during surgery is required, and blood pressure is the parameter most typically monitored for this purpose7. An arterial line allows for a continuous blood pressure measurement, which is more accurate than traditional non-invasive measurement8 as non-invasive techniques underestimate values during hypertension and overestimate them during hypotension7,8. An accurate reading of blood pressure during these experiments is fundamental to be able to control the amount of fluids and vasopressors that must be administered to the pig.
The Toronto Organ Preservation Laboratory has been using porcine models for more than 10 years and has standardized these procedures throughout the years with excellent outcomes. Although other approaches can be found in the literature for the same procedures, the goal of this paper is to present the techniques developed and perfected over the years at our facility.
Protocol
All animals used for this study received humane care in accordance with the "Principles of Laboratory Animal Care" formulated by the National Society for Medical Research and the "Guide for the Care of Laboratory Animals" published by the National Institutes of Health, Ontario, Canada. All studies were approved by the Animal Care Committee of the Toronto General Research Institute. In this study, 11-12-week-old male Yorkshire pigs weighing 30-40 kg were used.
1. Intubation
- House the pigs for at least 5 days before the procedure to allow them to adjust to the environment and reduce overall stress levels
- Fast the pig for 6 h before anesthesia.
- Anesthetize the pig with an intramuscular (IM) injection of ketamine (20 mg/kg) as a dissociative anesthetic, midazolam (0.15 mg/kg) for sedation, and atropine (0.04 mg/kg) to reduce salivation and bronchial secretions, in the housing facility.
- Give O2 and isoflurane at 5% with the help of a mask once the pig has fallen asleep for at least 5 min.
- Move the pig into a transportation cart and transfer to the operating room (OR).
- Place the pig in a supine position on the OR table.
- Place the ventilation mask with 2 L/min O2 and 5% isoflurane.
NOTE: Make sure the pig has inspiratory effort. - Position the oxygen saturation probe on the pigs' ear.
NOTE: The probe can also be placed on the tongue, tail, dewclaw3, lips, or inguinal fold. - Ask an assistant to place the electrocardiogram electrodes and blood pressure cuff.
NOTE: Electrodes are placed in the right axillary fold, left axillary fold, and left inguinal fold. The blood pressure cuff can be placed either in the foreleg or hindleg. - Check for relaxation of the jaw after 4-5 min of ventilation with isoflurane at 5%.
NOTE: if resistance is noted, continue ventilation with isoflurane for an additional 3 min or until the jaw is relaxed. - Ask the assistant to hold the mandible up, maxilla down, and tongue out of the way.
- Insert a laryngoscope with a straight miller blade down the midline and lift the epiglottis.
NOTE: Use an endotracheal tube to push down the soft palate and allow the epiglottis to pop up (Figure 1). - Visualize the vocal cords and spray them two times with lidocaine non-aerosol spray (10 mg/metered dose) to prevent spasms during intubation.
- Remove the laryngoscope and replace the oxygen mask for 30 s.
- Remove the oxygen mask, insert the Miller straight blade down the midline, and lift the epiglottis. Insert a 7.0 mm endotracheal tube beyond the vocal cords.
NOTE: If there is resistance or spasm of the vocal cords, repeat steps 1.13 and 1.14. - Hold the endotracheal tube in place and slide out the laryngoscope and stylet.
- Inflate the endotracheal tube cuff with 5-8 cc of air and connect it to a capnometry sensor to ensure the correct placement of the tube.
NOTE: Other methods to ensure proper placement include looking for chest drives, condensation in the endotracheal tube, and listening to bilateral breath sounds. - Turn on the ventilator and adjust to 15-20 breaths per minute, isoflurane to 2%-2.5%, tidal volume to 10-15 mL/kg of bodyweight, and peak inspiratory pressure between 18-20 cmH2O9,10,11.
- Fix the endotracheal tube with tape.
- Apply eye lube to prevent dryness and ulceration during the procedure.
- Ensure proper anesthetization by assessing the jaw tone and adjusting isoflurane accordingly. Rigid mandibular muscles indicate a light level of anesthesia3.
2. Central venous catheter placement
NOTE: The choice of catheter depends on the type of model being used. For survival models, a catheter that can be tunneled at the end of the procedure is used. For terminal models, a simpler model (refer to Table of Materials) is used.
- After intubation, with the pig in the supine position, disinfect both sides of the neck with either iodine or chlorhexidine solution.
- Prepare the catheter by flushing it with 5 to 10 mL of saline and ensure it does not have air inside. Place the dilator through the catheter.
- Inspect and test the guidewire to confirm proper aspects and function.
NOTE: The guidewire must move freely inside the sheath and must not have any defects or kinks. If any alteration is noted, replace the guidewire. - Identify the following three landmarks on the pig's neck: (1) the caudal ramus of the mandible, (2) the cranial manubrium, and (3) the cranial point of the right shoulder formed by the greater tubercle of the humerus6 (Figure 2).
- Insert the finder needle attached to a 5 mL syringe in the center of the triangle formed by the three landmarks at a 45° angle from the skin and directed toward the caudal side, while slightly pulling back on the syringe embolus.
- Once the jugular vein is found, remove the syringe from the finder needle and stabilize the needle while placing the thumb over the needle hub. Ensure the finder needle is secured in place.
NOTE: If blood coming from the needle is suspected to be arterial, remove the needle immediately and apply pressure for 5 min. - Feed the guidewire into the finder needle and advance into the external jugular vein (Seldinger's technique12). Minimal to no resistance should be met. Remove the finder needle and keep the guidewire in place.
NOTE: Use an ECG trace to make sure the guidewire does not go in too deep. If alterations in cardiac rhythm are noted, remove the guidewire slightly. - Insert the tip of an 11-blade into the puncture site and create an incision (around 0.5 cm) for the dilator and catheter.
- Feed the dilator and catheter over the guidewire to dilate the skin and the subcutaneous tissue. Once in place, remove the dilator from the catheter.
- Aspirate from the catheter lumen to ensure proper flow and flush with 5-10 mL of saline.
- Suture the catheter into the skin with a 0 silk suture and ensure the catheter is secure13.
3. Arterial line placement
NOTE: Carotid placement of the arterial line is preferred since it provides a more direct route to the heart, and the vessels of the neck are larger than the femoral vessels11. Preferably, the arterial line should be placed on the contralateral side of the central venous catheter and under direct vision; if this is not possible, it can be placed on the same side as the venous catheter.
- Make a vertical incision of 5-7 cm medial to the trachea using a cautery pen (Figure 3).
- Dissect the subcutaneous tissue and the plane between the cutaneous facii and colli muscles11.
- Dissect the facial plane between the trachea and the sternomastoideus muscle to localize and open the carotid sheath11 (Figure 4).
- Use blunt dissection to isolate the common carotid artery (Figure 5).
- Place two 2-0 silk ties around the carotid artery for vascular control and tie the distal one (Figure 6).
- Place the right angle forceps under the artery.
- Ask the assistant to hold the proximal tie and insert the angiocath needle tip into the carotid artery at a 45° angle. Once a flash of blood is seen, advance the needle slightly at a shallow angle and then advance the catheter only into the artery.
- Remove the needle while keeping the catheter in place.
- Attach the catheter to the arterial line, aspirate, and flush with 5-10 mL of saline to ensure there is no resistance.
- Place a tie around the catheter and artery to secure the catheter.
4. After surgery care (survival model)
- Tunnel the venous line catheter and fix it to the neck of the pig.
- Once the animal is hemodynamically stable and can be weaned off vasopressors, remove the arterial line catheter, tie the distal side of the carotid artery, and verify hemostasis. Close the wound with an uninterrupted suture.
- Move the pig to the transportation cart and position it in a sternal position.
- Turn off isoflurane and monitor the pig until signs of anesthesia recovery are present (movements and breathing against the ventilator).
- Turn off the ventilator once effective spontaneous respirations are noted, but keep the O2 supply at 2 L/min.
- Turn off the O2 supply after 10-15 min. The pig should be able to maintain a saturation of at least 94% on its own. If that is not the case, turn on the O2 again and reassess in another 5-10 min.
- Move the pig back to its pen once it maintains O2 saturation.
NOTE: The pig should be moved back to its pen with the endotracheal tube in place. - Remove the endotracheal tube once signs of swallowing and/or chewing are evident (between 2-4 h after the end of surgery).
- Monitor the pig in its pen and never leave it alone. Once the pig is extubated and can maintain a sternal position, it can be left unattended.
Results
Pig monitoring during surgery is indispensable, and the normal parameters expected during surgery are shown in Table 13,14. The approximate duration needed to perform each procedure is shown in Table 2.
As mentioned previously, this lab has been performing these procedures for the last 10 years. Over this period, 595 liver experiments, 277 kidney experiments, and 100 pancreas experiments have been carried out (Table 3).
The 100 pancreas experiments (for 151 pigs) were performed over the last 2 years and are used here as representative results.
Two animals presented a difficult airway, for which an experienced veterinary technician needed to perform the intubation. The rest of the procedure took place without any other complications. This translates to a 98.7% intubation success rate (Table 4).
Five animals were unfortunately lost over this period, related to central line placement procedures, which equals a 96.7% success rate (Table 4). Three of the animals presented arrhythmia after central line placement and progressed to asystole within seconds; no drugs were administered after, as due to the nature of the experiments, this was considered a warm ischemia time and may have affected the end results. An autopsy was performed on one of the animals; multiple clots were discovered in the right ventricle, as well as signs suggestive of a pulmonary embolism. The other two animals presented sudden cardiac death with no signs of prior arrhythmia. Upon autopsy, enlarged hearts and pleural edema were noted.
Arterial line placement is always performed, at this facility, after central venous line placement; as previously mentioned, five animals were lost during placement of the central venous line, before arterial line placement had even started. Arterial line placement was successful in the 146 remaining attempts, translating to a 100% success rate (Table 4).

Figure 1: Airway landmarks. An endotracheal tube is used to push down the soft palate and allow the epiglottis to pop up. Please click here to view a larger version of this figure.

Figure 2: Central venous catheter placement using the triangulation technique. Three landmarks on the pig's neck: the caudal ramus of the mandible, the cranial manubrium, and the cranial point of the right shoulder formed by the greater tubercle of the humerus. Please click here to view a larger version of this figure.

Figure 3: Arterial line placement. A mark showing the site of the vertical incision 5-7 cm medial to the trachea. Please click here to view a larger version of this figure.

Figure 4: Sternomastoideus and sternohyoideus muscle during dissection for arterial line placement. The facial plane between the trachea and the sternomastoideus muscle is dissected to localize and open the carotid sheath. Please click here to view a larger version of this figure.
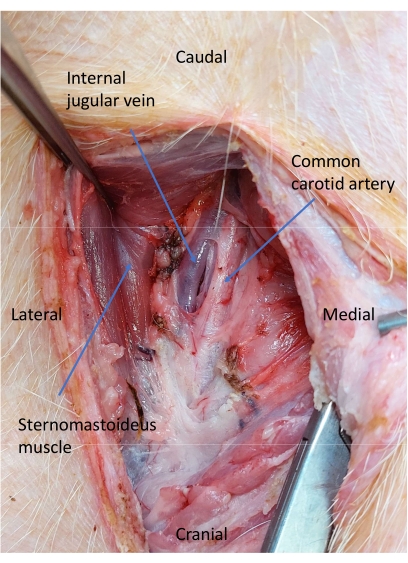
Figure 5: Jugular vein and carotid artery after dissection of the carotid sheath. A blunt dissection is performed to isolate the common carotid artery. Please click here to view a larger version of this figure.

Figure 6: Isolated common carotid artery. Two 2-0 silk ties are placed around the carotid artery for vascular control. Please click here to view a larger version of this figure.
| Parameter | Normal range |
| Temperature | 38–39.5 °C |
| End CO2 | 40–50 mmHg |
| Systolic blood pressure | 112–139 mmHg |
| Diastolic blood pressure | 72–98 mmHg |
| Mean arterial blood pressure | 86–123 mmHg |
| Heart rate | 70–100 bpm |
| Respiratory rate | 10–20 bpm. |
Table 1: Normal parameters to be maintained during surgery. The table lists the normal range of the parameters that need to be maintained during the surgery.
| Procedure | Time (min) |
| Intubation | 15 ± 5 |
| Central venous catheter | 20 ± 10 |
| Arterial line | 20 ± 10 |
Table 2: Duration of the procedures. The table lists the approximate duration required to perform intubation, central venous catheterization, and arterial line placement.
| Organ | Number of experiments |
| Liver | 595 |
| Kidney | 277 |
| Pancreas | 100 |
Table 3: Number of experiments per organ. The table lists the number of experiments performed on the liver, kidney and pancreas.
| Successful | Not successful | Success (%) | Failure (%) | |
| Intubations | 149 | 2 | 98.7 | 1.3 |
| Central line placement | 146 | 5 | 96.7 | 3.3 |
| Arterial line placement* | 146 | 0 | 100 | 0 |
| *All arterial line placements are performed after central line placement | ||||
Table 4. Success rate per procedure. The table lists the success rate of the intubations, central venous catheterizations, and arterial line placements performed on 151 pigs.
Discussion
Every research center must create its own protocols and guidelines for translational research models; however, some basic rules are to be followed to guarantee successful results.
First, the pig must be submitted to a physical examination as soon as it arrives at the housing facility. A total of 5-7 days of conditioning is necessary before the procedure to reduce stress levels and allow the pig to recover the weight lost during transportation3. It is important to note that we use pigs that come from a breeding farm and are not grown exclusively for research purposes, and as expected, health issues may arise throughout the year (seasonal viruses).
Prior to the procedure, the pig must be fasted for solid food for 6-8 h, however water is allowed until the time of surgery3. It is also especially important to remember that pigs are susceptible to anesthesia-induced cardiac arrhythmias. Placement of an ECG during the induction of anesthesia and vascular access helps to identify them, and antiarrhythmic drugs can be administered if necessary3. This has been an important reason for the loss of pigs during our experiments. A certified veterinarian or experienced veterinarian technician should be available in case the team encounters an issue that cannot be solved. The use of ultrasound is not frequent in our lab because of limited availability, so we reserve its use for difficult cases. Planning experiments around the availability of the ultrasound machine would significantly limit the number and timing of experiments. However, if broadly available in other research facilities, its usage is vastly encouraged.
The experience of the surgical team is also a key factor. All the research staff directly involved in the surgical part of the protocols, at this research facility, are trained general surgeons, and most also have training in HPB and/or transplantation surgery. The majority have no previous experience in translational porcine models. Each team consists of a senior and a junior fellow. The senior fellow has at least 1 year of experience in porcine translational models and oversees the training of the junior fellow in the basic techniques described in this paper. The junior fellow usually becomes proficient in 4 to 6 weeks.
Here are some tips that can be helpful in case of altered vital sign parameters during the experiments. Hypothermia is a common complication during surgery15; a heating pad can be placed on the surgery table, and a warming blanket system can also be placed on top of the pig. If these measures are not enough, the use of warmed IV solutions can be considered.
If end CO2 is below or above normal ranges, correct placement of the endotracheal tube and proper inflation of the cuff should be confirmed. The CO2 sensor should be checked to ensure it is properly connected and working. The anesthesia machine should be checked for any leaks, and carbon dioxide beads replaced if exhausted. It is also important to make sure that inspiratory flow, breaths per minute, and peak inspiratory pressure are all set correctly according to the weight of the pig.
If the pulse oximetry sensor is not working in one of the locations previously mentioned, first it should be ensured that the sensor is properly working, that the ventilator is on, that oxygen is set to 2 L/min, and that the pig has normal coloration. If all the above are normal, the sensor should be moved to another of the mentioned locations. If this doesn't work, a blood gas analysis can be taken to verify oxygen saturation.
In abdominal transplantation surgery, hypotension is a critical component of post-reperfusion injury16. Increasing IV fluid rate and the use of vasopressors (we use norepinephrine) are common. Norepinephrine has both a vasopressor and an inotropic effect, so the heart rate must be constantly monitored. It is also important to remember that vasoconstriction might compromise the blood flow to the recently transplanted organ17.
The above-described procedures are used for research in abdominal transplantation surgery; however, we believe that they can be adapted and used for other types of research. Swine models are invaluable for translational research, and as such, research teams must ensure humane treatment and not allow any pain or suffering to the animals.
Disclosures
The authors do not have anything to disclose.
Acknowledgements
None.
Materials
| Name | Company | Catalog Number | Comments |
| 0.9% Sodium Chloride Irrigation, USP | Baxter | JF7124 | Saline |
| Angiocath 16 GA 1.88 , 1.7 x 48 mm | BD | 381157 | |
| Atropine sulfate (8 mg/20 mL - 0.4 mg/mL) | West-Ward | 17733 | 20 mL vial |
| Cook TPN Single Lumen Cathether Set | Cook medical | G08132 | with 10 Fr peel away introducer set |
| Hickman 9.6 F single-lumen CV cathether | Bard | 600560 | |
| Laryngoscope | Heine | ||
| Midazolam (5 mg/mL) | Sandoz | 46237968 | 10 mL vial |
| Miller blade 4 | Heine | 185 mm blade length | |
| Narketan (Ketamine - 100 mg/mL) | Vetoquinal | 8-00223 | 50 mL vial |
| Nasal tracheal tube cuffed 7.0 mm I.D. | Covidien | 86450 | |
| Optixcare eye lube for dogs and cats | Aventix | 5914304 | 20 g |
| Percutaneous Sheath introducer set with integral hemostasis valve/side port for use -7.5 Fr. Catheters | Arrow | SI-09880 | 8.5 Fr, 10 cm, 0.035 inch dia. Spring wire guide |
| Universal Electrosurgical Pad: Split with cord | 3M | 9165 | |
| Valleylab Rocker Switch Pencil Holster | Covidien | E2515H | |
| Xylocaine 10% Spray | AstraZeneca | 73050036 | lidocaine (10 mg/metered dose) |
References
- Dehoux, J. P., Gianello, P. The importance of large animal models in transplantation. Frontiers in Bioscience: A Journal and Virtual Library. 12, 4864-4880 (2007).
- Ettrup, K. S., et al. Basic surgical techniques in the Göttingen minipig: Intubation, bladder catheterization, femoral vessel catheterization, and transcardial perfusion. Journal of Visualized Experiments. (52), e2652 (2011).
- Swindle, M. M. . Swine in the Laboratory Surgery, Anesthesia, Imaging, and Experimental Techniques. , (2015).
- Chum, H., Pacharinsak, C. Endotracheal intubation in swine. Lab Animal. 41 (11), 309-311 (2012).
- Prasanna, N., et al. Safety and efficacy of vasopressor administration through midline catheters. Journal of Critical Care. 61, 1-4 (2021).
- Flournoy, W. S., Mani, S. Percutaneous external jugular vein catheterization in piglets using a triangulation technique. Laboratory Animals. 43 (4), 344-349 (2009).
- Kuck, K., Baker, P. D. Perioperative noninvasive blood pressure monitoring. Anesthesia and Analgesia. 127 (2), 408-411 (2017).
- Pour-Ghaz, I., et al. Accuracy of non-invasive and minimally invasive hemodynamic monitoring: where do we stand. Annals of Translational Medicine. 7 (17), 421 (2019).
- Kaths, J. M., et al. Normothermic ex vivo kidney perfusion for the preservation of kidney grafts prior to transplantation. Journal of Visualized Experiments. (101), e52909 (2015).
- Parmentier, C., et al. Normothermic ex vivo pancreas perfusion for the preservation of pancreas allografts before transplantation. Journal of Visualized Experiments. (185), e63905 (2022).
- McCall, F. C., et al. Myocardial infarction and intramyocardial injection models in swine. Nature Protocols. 7 (8), 1479-1496 (2012).
- Graham, A. S., Ozment, C., Tegtmeyer, K., Lai, S., Braner, D. A. V. Central venous catheterization. The New England Journal of Medicine. 356 (21), 21 (2009).
- Taylor, R. W., Palagiri, A. V. Central venous catheterization. Critical Care Medicine. 35 (5), 1390-1396 (2007).
- Reed, R., et al. Accuracy of an oscillometric blood pressure monitor in anesthetized pigs. Laboratory Animals. 52 (5), 490-496 (2018).
- Musk, G. C., Costa, R. S., Tuke, J. Body temperature measurements in pigs during general anaesthesia. Laboratory Animals. 50 (2), 119-124 (2016).
- Aggarwal, S., Kang, Y., Freeman, J. A., Fortunato, F. L., Pinsky, M. R. Postreperfusion syndrome: Hypotension after reperfusion of the transplanted liver. Journal of Critical Care. 8 (3), 154-160 (1993).
- Manning, M. W., Kumar, P. A., Maheshwari, K., Arora, H. Post-reperfusion syndrome in liver transplantation-An overview. Journal of Cardiothoracic and Vascular Anesthesia. 34 (2), 501-511 (2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved