Method Article
A Standardized Murine Model of Extracorporeal Shockwave Therapy Induced Soft Tissue Regeneration
* These authors contributed equally
In This Article
Summary
This article describes a standardized murine model of tissue regeneration via shockwave treatment.
Abstract
Shockwave therapy (SWT) shows promising regenerative effects in several different tissues. However, the underlying molecular mechanisms are poorly understood. Angiogenesis, a process of new blood vessel formation is a leading driver of regeneration in softer tissues as well as a recently discovered effect of SWT. How the mechanical stimulus of SWT induces angiogenesis and regeneration and which pathways are involved is not fully understood. To further improve the clinical use of SWT and gain valuable information about how mechanical stimulation can affect tissue and tissue regeneration, a standardized model of SWT is needed. We, hereby, describe a standardized, easy to implement murine model of shockwave therapy induced regeneration, utilizing the hind-limb ischemia model.
Introduction
Shockwave therapy (SWT) was first introduced in clinical practice as a means of disintegrating kidney stones via extracorporeal application. In the 1990´s, an incidental finding of iliac crest thickening in X-ray recordings following repeated lithotripsy revealed a bone morphogenic effect of SWT1. This prompted a surge of new applications in orthopedic use. SWT, thereby evolved into an acknowledged treatment option for, long bone non-unions, lateral epicondylitis, as well as achilles tendonitis2,3,4,5. Recent evidence now again broadens the spectrum of appliances beyond orthopedics, into softer tissues and wound healing disorders6,7. Here studies could show effectiveness of SWT in a heterogeneous assembly of conditions including for example erectile dysfunction or spasticity after stroke8,9,10.
However, the molecular mechanisms underlying SWT are still not fully understood and require further research. With a focus on cardiovascular disease our previous work demonstrates a promising effect of SWT in a murine model of myocardial infarction. Thereby angiogenesis was discovered as a core driver of myocardial regeneration following SWT11.
Angiogenesis describes the development of new vessels through sprouting and splitting of preexisting vessels. In the case of injury these new vessels facilitate the restoration of blood flow to the damaged area and thereby regeneration12.
Angiogenesis, therefore, represents a hallmark of tissue regeneration and a potential explanation for SWT effects in softer tissues. However, regeneration is a complex process with numerous inductor and effector mechanisms. Albeit the possibility to investigate them in an isolated cell culture setting, animal models are best suited to emulate these complex processes. Hind limb ischemia is a well-established model to investigate angiogenesis and regeneration in vivo13. To support further research of the regenerative effect of SWT we hereby present a feasible, standardized, murine model of SWT in hind limb ischemia.
Protocol
The experiments were approved by the institutional animal care and use committee at Innsbruck Medical University and by the Austrian ministry of science (BMWF-66.011/0110-V/3b/2019).
1. Induction of anesthesia and operational set-up
- Prepare a suitable environment for animal procedures: sterilize equipment, disinfect surfaces, make use of disposable masks, isolation gowns and gloves.
- Sedate a 18-12-weeks old mouse (strain and sex depending on the experimental setting) in a chamber attached to an isoflurane vaporizer at 4%.
- Check for the sufficient sedation via pedal or pinna reflex as indicators for deep pain recognition.
- When the animal is sufficiently sedated, turn off isoflurane flow and administer analgesia and anesthetics as per the approved animal care and use protocol use e.g., ketamine hydrochloride (80 mg/kg body weight) as an anesthetic and xylazine hydrochloride (5 mg/kg body weight) as an analgetic intra-peritoneally.
NOTE: Prepare syringe with intra-peritoneal medication before placing the animal in anesthesia chamber. - Examine the depth of anesthesia 5 min after injection by assessing the pedal withdrawal reflex.
- Apply eye ointment (e.g., 0.5 g Retinolpalmitat) to avoid corneal damage.
- Remove hair in and in the proximity to the surgical area, in particular the left hind limb and groin. Depilatory cream can be used instead of razors or trimmers to avoid skin injury.
- Fixate the animal in a supine position with extended limbs on a heating plate using adhesive tape.
- Disinfect and clean the area of surgery with 10% povidone-iodine or similar disinfectant. Use sterile field drape.
2. Procedure
- Use a microscope between 10x and 20x magnification to perform the surgery.
- Make a skin incision (~1.5 cm) proximal to the knee joint using surgical scissors.
- Gently separate the skin from underlying tissue using blunt forceps.
- Identify the femoral vessels. Carefully separate artery, vein, and nerve using forceps and scissors.
- Starting proximally at the level of the inguinal ligament, carefully remove the surrounding connective tissue until the artery is optimally displayed. As a distal endpoint, the arterial branching into the saphenous and popliteal artery should be visible.
- Ligate the proximal femoral artery at the level of the inguinal ligament using a 7-0 polypropylene suture.
- Occlude the distal end of the femoral artery proximal to the branching into saphenous and popliteal artery using a 7-0 polypropylene suture.
- Excise the femoral artery segment between the distal and proximal knots using the diathermy.
NOTE: Excising the femoral artery by cutting with surgical scissor is also possible. However, using a diathermy occludes the vessel in addition to the suture in case the knots fail. - Make sure the femoral artery is safely occluded and no bleedings are visible in the field of operation.
NOTE: Narrow distance between skin sutures is recommended to avoid ultrasonic gel decontamination of the wound during SWT application. - Suture the skin incision using 5-0 non-absorbable nylon sutures with single knots.
- Disinfect the surgical area with cotton swabs.
3. Shockwave therapy application
- Make sure skin incision is fully closed.
- Define treatment parameters at shockwave device. In this experimental setting, an energy flux density of 0.1 mJ/mm2 at a frequency of 3 Hz for a total of 300 impulses was used.
NOTE: The energy levels were adopted from previous results14 utilizing focused extracorporeal shockwave treatment. - Apply ultrasonic gel to the treatment area on the inner thigh for proper coupling.
- Make sure no air bubbles are trapped within the gel.
NOTE: Proper coupling with enough gel is essential for adequate SWT application. Small air bubbles within the gel will absorb shockwaves and decrease their effect. - Apply 300 impulses by toggling the foot switch while slowly moving the applicator over the thigh.
NOTE: If SWT is not applied immediately after surgery, avoid possible shockwave energy absorption due to re-grown hair by removal prior to treatment. - After treatment, wipe off any residual ultrasonic gel to prevent cooling of the thigh.
- Move the animal into a recovery cage exposed to a heating lamp to avoid hypothermia.
- Monitor the animal carefully until awake and administer a dose of 0.05 mg/kg body weight of buprenorphine, subcutaneously for adequate analgesia.
- Monitor the health and wellbeing of animals daily until the surgical incision is healed fully.
NOTE: Treatment can be limited to one session or be repeated multiple times. In this example, a single application was performed.
4. Blood flow measurement
- Perform blood flow measurement immediately after surgery and at various timepoints afterwards depending on the experimental setting.
- Sedate the animal in a chamber attached to an isoflurane vaporizer at 4%.
- When the animal is sedated, turn off isoflurane flow and administer anesthetics and analgesics. As per the approved animal care and use protocol apply ketamine hydrochloride (80 mg/kg body weight) and xylazine hydrochloride (5 mg/kg body weight) intra-peritoneally.
- Examine the depth of anesthesia 5 min after the injection by assessing the pedal withdrawal reflex.
- Utilize eye ointment (e.g., 0.5 g Retinolpalmitat) to avoid corneal damage.
- Fixate the animal in a supine position with extended limbs on a heating plate using adhesive tape.
- Remove hair from both hind limbs meticulously.
- Measure limb perfusion via laser doppler according to manufacturer's instruction.
NOTE: The ratio of ischemic limb versus non-ischemic limb blood flow should be used as the prime parameter.
Results
Utilizing this protocol significant differences in hind limb perfusion can be observed and monitored after SWT intervention. Representative images show a marked difference in limbs treated with SWT (Figure 1B) compared to untreated control limbs (Figure 1A). Here, perfusion is portrayed via thermal flaring with cold colors representing low perfusion and warm colors representing high perfusion. Quantification of laser doppler readings show a significant increase in perfusion 4 weeks after surgery. (Figure 1C), Concomitantly less necrosis can be observed in SWT treated animals (Figure 1D). Necrosis was assessed as described previously16.
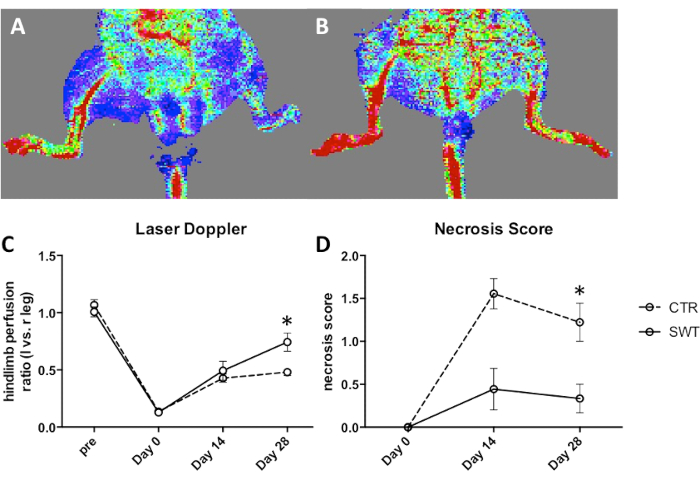
Figure 1: Improvement of blood perfusion upon shock wave therapy in a murine model of hind limb ischemia. Representative laser doppler images of (A) untreated animal and (B) ischemic limbs 4 weeks after SW. (C) Quantification of weekly-performed laser doppler imaging revealed increased limb perfusion upon SWT after 4 weeks. Blood flow is expressed in the ratio of ischemic limb versus not ischemic limb. *p < 0.05. (D) Evaluation of necrosis shows a significant improvement in animals treated with shockwave therapy after 4 weeks. *p < 0.05. This figure has been modified from Holfeld et al15. CTR = untreated control, SWT = shockwave therapy. Results are expressed as mean ± SEM (standard error of the mean). Statistical comparisons were performed by student's t-test. P-values <.05 were considered statistically significant. Please click here to view a larger version of this figure.
Discussion
Shockwave treatment shows promising results in several soft tissue regeneration settings. However, to further augment, improve or isolate these regenerative capability's, first the basics of SWT induced regeneration should be uncovered on a molecular level. Tissue regeneration is complex and involves many biological processes including, innate and acquired immunity, inflammation, cell cycle progression, apoptosis, cellular differentiation, angiogenesis and others17,18. Isolated mechanisms of SWT may be studied in vitro, utilizing a water bath application, but fall short to comprehensively emulate in vivo regeneration. Thereby the correct investigation of Pathways activated by SWT can only be achieved in vivo.
The hind limb ischemia mouse model is well established, easy to implement. Additionally, it shows a low mortality rate and a low severity compared to other surgical means to investigate tissue regeneration. Furthermore, the hind limb ischemia model provides easy access to treated tissue for tissue collection or other means of evaluation (e.g., ultrasonic evaluation, laser doppler etc.). This model has the following limitations. One major limitation is the acute nature of the ischemia induced by removing the femoral artery while most ischemic diseases are chronic processes. Further, due to young age and healthy collateral tissue, rodents tend to heal to a great extent after ischemia even without therapeutic interventions.
Evidence for the effects of SWT are mostly gained through medical studies but usually lack in-depth research and evaluation of molecular mechanisms. A standardized protocol could, thereby present a means for researchers to compare their work surrounding SWT regeneration. In this regard, this protocol was designed to represent a modifiable foundation, easily adjustable to different tissues, SWT applicators, treatment regimes, or readouts. Accordingly, only a few steps in this protocol can be deemed crucial (see below). This protocol thereby presents an easy, feasibly, and standardized way to induce and study regeneration via shockwave therapy in vivo.
Crucial Steps
As in all animal models, it is crucial to avoid infections, unnecessary suffering of the animals and promote reproduceable clean data. Therefore, instruments should be disinfected properly. All work including research animals should be performed by capable, trained individuals. Insufficiency of either mentioned points must be avoided. Make sure not to mix up the femoral vein with artery. Avoid thermal muscle injury while using the diathermy, as it might bias the blood flow results.
Familiarization with this tool before usage in an animal model is highly recommended. Make sure not to affect the surrounding tissue, by double checking for quenched tissue in the forceps part of the diathermia prior to activation. When conducting SWT keep in mind that different SWT devices work differently and that therapy should be conducted in accordance with the user manual of the used device.
Disclosures
Holfeld J. and Grimm M. are shareholders of Heart Regeneration Technologies GmbH, an Innsbruck Medical University spin-off aiming to promote cardiac shockwave therapy (www.heart-regeneration.com). All other authors have nothing to disclose.
Acknowledgements
This study was supported by an unrestricted AUVA research grant to JH and CGT.
Materials
| Name | Company | Catalog Number | Comments |
| 10% Povidone | |||
| 5-0 Nylon suture | Ethicon Inc. | ||
| 7-0 silk suture | Ethicon Inc. | ||
| Cautery | Martin | ME-102 | |
| depilatory cream | Nivea | ||
| Gauze | Gazin | ||
| Heating Plate | |||
| Ketamine hydrochloride | anesthesia | ||
| Laser Doppler | Moor instruments | ||
| Surgical Tools | Fine Science Tools | ||
| Xylazine hydrochloride | anesthesia |
References
- Schaden, W., et al. Extracorporeal shockwave therapy (ESWT) - First choice treatment of fracture non-unions. International Journal of Surgery. 24, 179-183 (2015).
- Xu, Z. H., et al. Extracorporeal shock wave treatment in nonunions of long bone fractures. International Orthopaedics. 33, 789-793 (2009).
- Melegati, G., Tornese, D., Bandi, M., Rubini, M. Comparison of two ultrasonographic localization techniques for the treatment of lateral epicondylitis with extracorporeal shock wave therapy: A randomized study. Clinical Rehabilitation. 18, 366-370 (2004).
- Zhang, S., Li, H., Yao, W., Hua, Y., Li, Y. Therapeutic response of extracorporeal shock wave therapy for insertional achilles tendinopathy between sports-active and nonsports-active patients with 5-year follow-up. Orthopedic Journal of Sport Medicine. 8, 1-6 (2020).
- Dedes, V., et al. Effectiveness and safety of shockwave therapy in tendinopathies. Materia Socio Medica. 30, 141 (2018).
- Surace, S. J., Deitch, J., Johnston, R. V., Shock Buchbinder, R. wave therapy for rotator cuff disease with or without calcification. Cochrane Database of Systematic Reviews. 3 (3), 008962 (2020).
- Mittermayr, R., et al. Extracorporeal shock wave therapy (ESWT) for wound healing: Technology, mechanisms, and clinical efficacy. Wound Repair Regeneration. 20, 456-465 (2012).
- Fode, M., Hatzichristodoulou, G., Serefoglu, E. C., Verze, P., Albersen, M. Low-intensity shockwave therapy for erectile dysfunction: Is the evidence strong enough. Nature Reviews Urology. 14, 593-606 (2017).
- Guo, P., et al. Positive effects of extracorporeal shock wave therapy on spasticity in poststroke patients: a meta-analysis. Journal of Stroke and Cerebrovascular Diseases. 26 (11), 2470-2476 (2017).
- Vardi, Y., Appel, B., Jacob, G., Massarwi, O., Gruenwald, I. Can low-intensity extracorporeal shockwave therapy improve erectile function? A 6-month follow-up pilot study in patients with organic erectile dysfunction. European Urology. 58, 243-248 (2010).
- Gollmann-Tepeköylü, C., et al. miR-19a-3p containing exosomes improve function of ischaemic myocardium upon shock wave therapy. Cardiovascular Research. 116 (6), 1226-1236 (2019).
- Otrock, Z. K., Mahfouz, R. A. R., Makarem, J. A., Shamseddine, A. I. Understanding the biology of angiogenesis: Review of the most important molecular mechanisms. Blood Cells, Molecules and Diseases. 39, 212-220 (2007).
- Ahn, H., et al. A murine model of hind limb ischemia to study angiogenesis and arteriogenesis. Physiology and Behavior. 176, 139-148 (2017).
- Pölzl, L., et al. Defining a therapeutic range for regeneration of ischemic myocardium via shock waves. Science Reports. , 409 (2021).
- Holfeld, J., et al. Low energy shock wave therapy induces angiogenesis in acute hind-limb ischemia via VEGF receptor 2 phosphorylation. PLoS One. 9, 1-7 (2014).
- Theurl, M., et al. The neuropeptide catestatin acts as a novel angiogenic cytokine via a basic fibroblast growth factor-dependent mechanism. Circulation Research. 107 (11), 1326-1335 (2010).
- Noonan, D. M., De Lerma Barbaro, A., Vannini, N., Mortara, L., Albini, A. Inflammation, inflammatory cells and angiogenesis: Decisions and indecisions. Cancer Metastasis Reviews. 27, 31-40 (2008).
- Aurora, A. B., Olson, E. N. Immune modulation of stem cells and regeneration. Cell Stem Cell. 15, 14-25 (2014).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved