Method Article
Development of a Cadaveric Multiport Model of Posterior Circulation Aneurysm Clipping for Neurosurgery and Otolaryngology Residents
In This Article
Summary
A model protocol to train neurosurgery and otolaryngology resident learners on endoscopic transclival clipping of posterior circulation aneurysms is described. Two endoscopic approaches to access the silicone-injected or perfused posterior circulation of cadaveric heads are established for training. Learners are tasked with clipping of posterior circulation based on clinical scenarios.
Abstract
Posterior circulation aneurysms are difficult to treat with the current methods of coiling and clipping. To address limitations in training, we developed a cadaveric model to train learners on endoscopic clipping of posterior circulation aneurysms. An endoscopic transclival approach (ETA) and a transorbital precaruncular approach (TOPA) to successfully access and clip aneurysms of the posterior circulation are described. The model has flexibility in that a colored silicone compound can be injected into the cadaveric vessels for the purpose of training learners on vascular anatomy. The other option is that the model could be connected to a vascular perfusion pump allowing real-time appreciation of a pulsatile or ruptured aneurysm. This cadaveric model is the first of its kind for training of endoscopic clipping of posterior circulation aneurysms. Learners will develop proficiency in endoscopic skills, appropriate dissection, and appreciation for relative anatomy while developing an algorithm that can be employed in a real operative arena. Going forward, various clinical scenarios can be developed to enhance the realism, allow learners from different specialties to work together, and emphasize the importance of teamwork and effective communication.
Introduction
Treatment of posterior circulation aneurysms presents unique challenges and has higher complication rates compared to other cerebral aneurysms1. Transcranial clipping of posterior circulation aneurysms is technically challenging, with high complication rates and morbidity2. Endovascular coiling and endoscopic endonasal surgery are safe alternatives, as they reduce complication rates and limit traction on the cerebrum3. Endovascular coiling has been shown to have benefits over open skull base approaches, and most centers now use an endovascular approach to treat cerebral aneurysms4. However, many posterior circulation aneurysms are not amenable to coiling due to the location, vessel tortuosity, and vessel size2. Recent studies have shown the feasibility of using endoscopic approaches for the clipping of posterior circulation aneurysms5,6,7,8.
Although endoscopic endonasal surgery has demonstrated benefits over more invasive procedures, several studies document a learning curve associated with the use of endoscopic equipment9,10,11. It is this learning curve and lack of surgeon training and experience that limit the use of this safe and beneficial treatment option3. As endoscopic clipping for aneurysms is revealing itself to be a feasible and a safe treatment course, neurosurgery and otolaryngology residents will need to develop these surgical skill sets during their training. This need for technical skill combined with a steep learning curve necessitates the development of realistic training models, as several repetitions are needed to reduce operative room time and complication rate in endoscopic endonasal surgery9,11. In a human placental model of cerebral aneurysm clipping, Belykh et al. have demonstrated improvement in the use of aneurysm clip applicators in learners after simulation12. Similarly, training with 3-dimensional printed models has been shown to improve learner technical skills in aneurysm clipping13. As with any training model, cost-effectiveness and reproducibility are leading objectives for wider accessibility. We have previously demonstrated the utility of an ETA and a TOPA in a cadaver model of posterior circulation aneurysm clipping, with approach access and visualization affected by clip location14. TOPA can be used in conjunction with endoscopic endonasal approaches, and has previously demonstrated shorter working distances, improved visualization, and angles resulting in increased access to structures4,14. The TOPA procedure is a new approach for clip ligation of aneurysms, and its applicability can be further explored via simulation for access to both tumors and aneurysms. In this protocol, we present the steps for development of a realistic, cost-effective, reproducible posterior circulation aneurysm-clipping model using ETA and TOPA as options to train neurosurgery learners. An advantage of our model is learner's exposure to authentic physical anatomy, with the option to incorporate realistic dynamic bleeding in the training of aneurysm clipping. This model can be set up with a static (silicone compound infused) or a dynamic (perfused) anatomy and is applicable to train neurosurgery or otolaryngology learners at various levels of expertise on the anatomy and management of posterior circulation aneurysms.
Protocol
In the development of this model, three cadaveric heads were obtained through the Oregon Health & Science University Body Donation Program and handled per the Code of Ethics approved by the Oregon Health & Science University Institutional Review Board.
1. Head Preparation
- Obtain fresh cadaveric heads. Secure the head in the sink, resting on a block with the neck facing upwards.
- Prepare a 5 L 1:100 solution of anticoagulant citrate dextrose and warm water. Use the solution within 72 h.
- Insert a 5-mm arterial cannula into the right jugular vein (lateral to carotid) and apply an arterial cannula clamp around the vessel. Perfuse the vessel for 15 min with a perfusion pump and anticoagulant citrate dextrose solution to wash the vessel. Remove the cannula and repeat with the left jugular vein and carotid arteries bilaterally. Repeat with a 3-mm cannula on the vertebral arteries bilaterally.
- Allow the heads to sit and dry overnight. Position the heads with the face facing upwards and neck positioned at a 45 ° angle with a supporting block underneath. Then store in a cold room at 5 °C overnight.
- Embed the heads in 2 L of embalming solution. Store heads in a bucket, submerged in 10% formalin fixative solution.
- Use the prepared heads with either silicone compound injections to train with static anatomy (step 2) or arterial injury and perfusion setup to train with dynamic anatomy (step 4).
- To complete the silicone compound injections (step 2): complete step 2, then move to step 3 to complete the dissection.
- To complete the arterial injury and perfusion setup (step 4): complete the step 3 dissection, then move to step 4 for perfusion instructions.
2. Silicone Compound Injections
- Prepare the silicone compound solution according to the manufacturer's instructions (50 µL diluent, 40 µL red silicone compound dye, and 4.5 µL curative agent).
- Use dissection to identify the external carotid arteries. Clamp the right external carotid artery with a hemostat to focus the injection into the intracerebral vasculature.
- Insert the arterial cannula into the right internal carotid artery and apply the arterial cannula clamp around the vessel. Inject 20 µL of silicone compound solution. Stop the injection when the flow of the silicone compound solution is noted at the left common carotid artery.
- Clamp the common carotid arteries bilaterally with hemostats.
- Insert an arterial cannula into the right vertebral artery and apply an arterial cannula clamp around the vessel. Inject 10 µL of silicone compound solution. Stop the injection when the flow of silicone compound solution is noted at the left vertebral artery.
- Clamp the vertebral arteries bilaterally with hemostats.
- Allow the head to sit at room temperature for 30 min, then gently remove all clamps.
3. Tissue Dissection
- Position heads with the face upwards and the neck positioned at a 45° angle with a block positioned beneath it in order to visualize the nasal passages.
- Obtain 0- and 30-degree endoscopes of 4 mm diameter and 18 cm length. Connect it to a fiber optic camera and a light source.
- Prepare all heads with both ETA and TOPA dissections.
- ETA 14 :
NOTE: See Figure 1A for representation of the ETA dissection.- Use a speculum to move the middle turbinates laterally on bilateral sides.
- Use suction and a Penfield 1 dissector to identify the sphenoid ostium bilaterally.
- With an 11-blade knife, make an incision on the right side of the nasal septum at the articulation of the sphenoid rostrum and vomer to bone. Make the incision 1 cm below the cribriform plate and to the floor of the nasal cavity.
- Dissect the mucoperiosteum from the bone using a Penfield 1 dissector.
- Disarticulate the sphenoid rostrum from the bony nasal septum with a Penfield 1 dissector and perform septectomy.
- Use a Kerrison 2 to cut the bone from the sphenoid ostia to the floor of the sphenoid sinus bilaterally.
- Grasp and remove the keel with a large pituitary rongeur to expose the sphenoid sinus, and remove mucosa.
- Use a trans sphenoidal drill to drill the remaining inferior rostrum of the sphenoid flush with the floor of the sphenoid sinus.
- Identify the bony impressions of the cavernous carotid arteries, opticocarotid recess, sella, and clivus (Figure 2); drill the clivus to expose its dura.
- Retract the pituitary gland (which should be in clear view) and drill out the clivus using a 5-mm coarse diamond burr.
- Remove the posterior clinoid.
- Dissect the lateral pituitary gland dura free from the pituitary gland; it is not necessary to dissect the dura over the pituitary gland.
NOTE: If any inadvertent pituitary gland dura dissection occurs, it will not impact the function of the model. - Use a Kerrison to remove bone fragments to expose the clival dura.
- Excise the clival dura to expose the basilar artery, anterior inferior cerebellar arteries (AICA), superior cerebellar arteries (SCA), and posterior cerebral arteries (PCA) (overview in Figure 3, anatomy detailed in Figure 4).
NOTE: The clival dura may be retracted with a U-based flap and stabilized with a 2-0 silk suture.
- TOPA 14 :
Note: See Figure 1B for representation of the TOPA dissection.- Move the caruncle laterally using forceps, then use scissors to make an incision medial to the caruncle where the conjunctiva meets the skin.
- Use iris scissors to widen the incision superiorly and inferiorly: the incision should continue behind the superior and inferior limbs of the medial canthal tendon; perform blunt dissection along the posterior limb of the medial canthal tendon to the posterior lacrimal crest.
- Use a Penfield 1 dissector to make an incision in the periosteum posterior to the lacrimal crest.
- Lift the periosteum from the medial orbit wall; continue to lift the periosteum toward the orbit apex in the subperiosteal plane.
- Identify and divide the anterior and posterior ethmoid arteries along the fronto-ethmoidal suture.
- Infracture the lamina papyracea between the ethmoid arteries and below the fronto-ethmoid suture with gradual pressure from a joker.
- Use a pituitary rongeur to remove bone in order to expose the sella, clivus, contralateral opticocarotid recess, and cavernous carotid arteries.
4. Arterial Injury & Perfusion Setup
- Prepare arterial injury.
- Use an endoscope to visualize the anatomy of the posterior circulation (representation in Figure 3 and Figure 4).
- Select the location for the desired bleeding. For possible locations for simulated aneurysm bleed see Figure 5. Make a 3-mm laceration in the desired artery.
- Perfusion setup & bleeding
- Cannulate the common carotid artery and secure with a clamp. Connect the cannula to the perfusion pump.
- Prepare artificial blood with a 3:1 ratio of water to commercially purchased fake blood. Then add 2 drops of red coloring for every 250 mL of water.
NOTE: Prepared artificial blood may be reused for multiple simulations. 3 L of blood is prepared for each cadaveric head prior to the start of the simulation. This can be stored at room temperature. Shake before use. - Start the perfusion pump and measure the mean arterial pressure (MAP) delivered to the cadaver head via an arterial line transducer and vital signs monitor. Maintain MAPs within a range of 65-110 mmHg to produce realistic bleeding depending on the desired simulation scenario.
5. Clip Placement in Simulation Training
- Use an aneurysm clip and clip applier for vessel clipping in this model (Figure 6).
- Use a clip applicator for clip placement (Video 1) at the simulated aneurysm sites of the posterior circulation (Figure 5).
Results
This model presents learners with multiple clinically-relevant sites for posterior circulation clipping, with either static (silicone compound-injected) or dynamic (perfused) options for training. Once dissection is complete, the investigators may use ETA and TOPA to provide learners with improved visualization of the posterior circulation14. Overview of ETA and TOPA are illustrated in Figure 1. For success of the model, investigators should complete the dissection protocol to expose posterior circulation clipping sites. Figure 2 details relevant anatomy to guide dissection. An endoscopic image of the completed dissection is shown in Figure 3, with relevant anatomy detailed in Figure 4. For investigators using the dynamic perfusion model, a small incision can be made at the desired clipping site and carotid arteries can be perfused via pump to produce simulated bleeding. Possible clip application sites for training are detailed in Figure 5. During simulation, learners are tasked with applying clips to obtain hemostasis. Representative images of endoscopic clip application using both ETA and TOPA methods are shown in Figure 6. A video representation of clip application to the SCA is shown in Video 1.
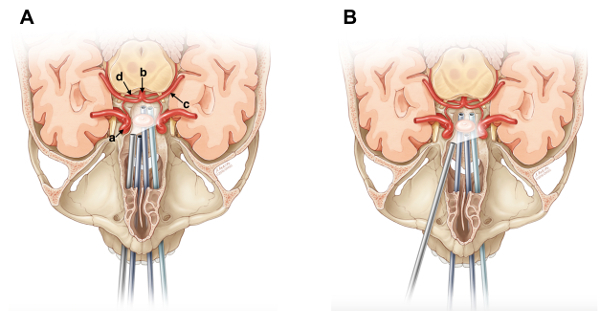
Figure 1: Representation of ETA(A)and TOPA (B). Results obtained after completion of Protocol step 3. (a) Right cavernous carotid artery. (b) Basilar artery. (c) Left PCA. (d) Right SCA. This figure has been modified from Ciporen et al. with permission4. Please click here to view a larger version of this figure.
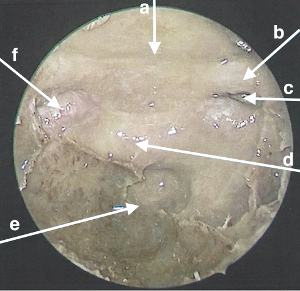
Figure 2: Endoscopic view and anatomy of ETA dissection. (a) Tuberculum sella. (b) Left optic nerve. (c) Left opticocarotid recess. (d) Sella. (e) Clivus. (f) Right cavernous carotid artery. Please click here to view a larger version of this figure.
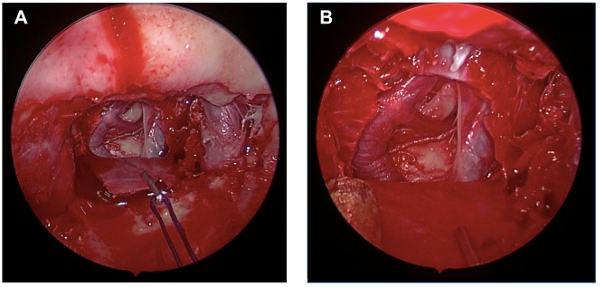
Figure 3: Image of completed posterior circulation exposure via the endoscope. Results obtained after completion of Protocol step 3. (A) Overall endoscopic view. (B) Close-up image of exposed posterior circulation. Please click here to view a larger version of this figure.
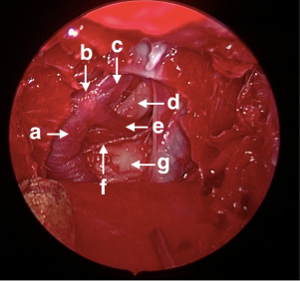
Figure 4: Endoscopic view and anatomy of the exposed posterior circulation. Visible after completion of Protocol step 3. (a) Basilar artery. (b) Basilar apex. (c) Left PCA. (d) Left Cranial nerve III. (e) Left SCA. (f) Left AICA. (g) Brainstem. Please click here to view a larger version of this figure.

Figure 5: Description of applicable posterior circulation clipping sites. Clips may be applied at the indicated locations for training purposes: basilar tip, SCA, PCA, AICA. Please click here to view a larger version of this figure.

Figure 6: Endoscopic images of sample clip application using ETA and TOPA methods. First panel: ETA approach to SCA clip application. (A) Application of clip to the left SCA. (B) Clip applied to the left SCA. Second panel: ETA + TOPA approach to SCA clip application. (C) Application of clip to the left SCA. (D) Clip applied to the left SCA. Please click here to view a larger version of this figure.

Video 1: Aneurysm clip application to left SCA. This figure has been modified from Ciporen et al. with permission4. Please click here to view this video. (Right-click to download.)
Discussion
Posterior circulation aneurysms have been historically hard to clip or coil, especially those originating off the SCA and AICA. Several techniques have been tried, such as endovascular pipeline embolization devices, microsurgical skull base approaches, and the supraorbital keyhole approach for clip application15,16,17. While these techniques are successful in some cases, the widespread applicability is limited due to stark differences in patient anatomy and the difficult to access posterior location of the vessels. This has led to the increased use of flow diversion in aneurysms deemed unclippable, untrappable, and uncoilable18. While flow diversion can lead to some aneurysmal obliteration, some aneurysms remain patent and therefore have a risk of rupture. We present, in this paper, the combined ETA + TOPA approach model, which may be an alternative for treating posterior circulation aneurysms.
Utilizing cadaveric models provides a multitude of advantages in training learners about the details of the procedure and enhancing competence in real-life management. First, variants in the anatomy can be readily appreciated through the utilization of silicone compound injections. These injections on cadaveric heads allow for appreciation of realistic anatomy. The silicone compound provides an excellent visualization of the vessel anatomy (both arterial and venous), which can be appreciated as the learner performs the dissection procedure. This dissection also allows for the authentic tactile feedback that may not be available in other simulated models19,20. It is important for the learner to localize the vessels early in order to avoid injuring them during dissection. Second, by providing cadaveric heads connected to a perfusion pump, the learner is able to develop the skill sets and algorithm for how to manage a pulsatile aneurysm or aneurysm rupture. This type of simulated training has been extremely successful in the cardiovascular literature for abdominal aortic aneurysms. Tomee et al. showed that learners who were able to develop an algorithm for aneurysm rupture during simulation had significantly lower death rates in real life21. Similarly, simulated cadaveric training will allow learners to have a go-to standard and alternatives to apply for hard to treat posterior circulation aneurysms.
Middle cerebral artery aneurysm clipping models have been developed using both cadaveric heads and human placentas22. The ETA + TOPA model is the first cadaveric human model developed for posterior circulation aneurysms, which offers a unique learning environment. Other animal models, virtual reality models, and cranial base models have been employed for the posterior circulation but are limited in the lack of ability for learners to appreciate and visualize human anatomical variants23,24,25. Middle cerebral artery aneurysms are often treated using open approaches with subsequent clipping. Posterior aneurysms, however, are more accessible via endoscopic procedures. The ETA + TOPA model provides a skill training experience in endoscopic neurosurgical procedures. Jukes et al. showed that endoscopic neurosurgical procedures require a different type of training and have unique stressors26. By providing a realistic learning environment, learners can drastically reduce the learning curve and feel a sense of confidence when entering the operative arena.
Critical steps in this protocol include proper silicone injection to allow proper visualization of anatomy, tissue dissection to view vasculature and generate a standardized model between learners, and maintenance of physiologic blood pressure range. These critical steps when performed properly ensure a model that more closely mimics realistic physiologic and anatomic parameters. Check for silicone leaks during injection. If leaks are present, remove the arterial clamp, advance the arterial cannula and re-clamp the vessel. If there is difficulty in titrating perfusion target blood pressure goals, re-zero the arterial line and repeat the perfusion. One of the limitations of this model is tissue consistency, as the embalming process may alter the tactile feedback during simulation. Additionally, although the TOPA method used in this model has been published previously, it is not yet an established method for clip ligation of aneurysms and warrants future training via simulation.
Going forward, improvements to the simulation experience can be provided. This will focus on altering various clinical scenarios in order to make the experience realistic to what is frequently seen in the operating room. Furthermore, neurosurgery and learners can work with anesthesia and otolaryngology residents in a team-based training. By providing team scenarios, learners will become more comfortable working with colleagues from different disciplines in the operating room. Instruction about effective communication and teamwork can be provided through debriefing sessions and focused instructor to provide feedback. These communication techniques will be applicable to a broad range of procedures and operations.
Disclosures
The authors have nothing to disclose relevant to this study.
Jeremy N. Ciporen, MD Consultant Spiway
Acknowledgements
The authors have no acknowledgements.
Materials
| Name | Company | Catalog Number | Comments |
| Anticoagulant citrate dextrose | Pierce Laboratories | 117037 | |
| Embalming solution | Chemisphere | ||
| 10% Formalin fixative | Chemisphere | B2915DR55 | |
| Red Microfil solution | Flow Tech | MV-130 | Silicone compound |
| Arterial cannula clamp | |||
| 5 mm Arterial cannula | Instrument Design & Mfg. Co. | ART187-2-CT | Used for jugular vein and carotid artery cannulation |
| 3 mm Arterial cannula | Instrument Design & Mfg. Co. | Used for vertebral artery cannulation | |
| Curved hemostat | Aesculap | BH139R | |
| Zero-degree endoscope (4 mm diameter, 18 cm length) | Karl Storz | H3-Z TH100 | |
| 30-degree endoscope (4 mm diameter, 18 cm length) | Karl Storz | ||
| Suction - 7 and 10 FR | V. Mueller | ||
| 11-blade surgical blade | Bard-Parker | 371111 | |
| Penfield 1 | Jarit | 285-365 | |
| Kerrison rongeur | Aesculap | FM823R, 3mm/180 mm | |
| Pituitary rongeur | Aesculap | FF806R | |
| Transsphenoidal drill | Depuy-Synthes | ||
| 5 mm coarse diamond burr drill | Depuy-Synthes | ||
| Forceps | Jarit | Carb bite I22-500 | |
| Iris scissors | Black & Black | B 66110 | |
| Perfusion Pump | Belmont Instrument Corporation, Billerica, MA, USA | Belmont Fluid Management System 2000 | |
| L-aneurysm clip | Peter Lazic Microsurgical Innovations | 45.782 | |
| Vessel clip system | Peter Lazic Microsurgical Innovations | 45.442 | |
| Dural flap clip | Weck | 523242 |
References
- Spetzler, R. F., et al. The Barrow Ruptured Aneurysm Trial: 6-year results. J Neurosurg. 123 (3), 609-617 (2015).
- Somanna, S., Babu, R. A., Srinivas, D., Narasinga Rao, K. V., Vazhayil, V. Extended endoscopic endonasal transclival clipping of posterior circulation aneurysms--an alternative to the transcranial approach. Acta Neurochir (Wien). 157 (12), 2077-2085 (2015).
- Yildirim, A. E., Divanlioglu, D., Karaoglu, D., Cetinalp, N. E., Belen, A. D. Pure Endoscopic Endonasal Clipping of an Incidental Anterior Communicating Artery Aneurysm. J Craniofac Surg. 26 (4), 1378-1381 (2015).
- Ciporen, J., Lucke-Wold, B., Dogan, A., Cetas, J. S., Cameron, W. E. Dual Endoscopic Endonasal Transsphenoidal and Precaruncular Transorbital Approaches for Clipping of the Cavernous Carotid Artery: A Cadaveric Simulation. J Neurol Surg B Skull Base. 77 (6), 485-490 (2016).
- Szentirmai, O., et al. Endoscopic endonasal clip ligation of cerebral aneurysms: an anatomical feasibility study and future directions. J Neurosurg. 124 (2), 463-468 (2016).
- Drazin, D., Zhuang, L., Schievink, W. I., Mamelak, A. N. Expanded endonasal approach for the clipping of a ruptured basilar aneurysm and feeding artery to a cerebellar arteriovenous malformation. J Clin Neurosci. 19 (1), 144-148 (2012).
- Ensenat, J., et al. Endoscopic endonasal clipping of a ruptured vertebral-posterior inferior cerebellar artery aneurysm: technical case report. Neurosurgery. 69, 127-128 (2011).
- Yoshioka, H., Kinouchi, H. The Roles of Endoscope in Aneurysmal Surgery. Neurol Med Chir (Tokyo). 55 (6), 469-478 (2015).
- Shikary, T., et al. Operative Learning Curve after Transition to Endoscopic Transsphenoidal Pituitary Surgery. World Neurosurg. , (2017).
- Chi, F., et al. A learning curve of endoscopic transsphenoidal surgery for pituitary adenoma. J Craniofac Surg. 24 (6), 2064-2067 (2013).
- Qureshi, T., et al. Learning curve for the transsphenoidal endoscopic endonasal approach to pituitary tumors. Br J Neurosurg. 30 (6), 637-642 (2016).
- Belykh, E., et al. Construct Validity of an Aneurysm Clipping Model Using Human Placenta. World Neurosurg. 105, 952-960 (2017).
- Mashiko, T., et al. Training in Cerebral Aneurysm Clipping Using Self-Made 3-Dimensional Models. J Surg Educ. 74 (4), 681-689 (2017).
- Ciporen, J. N., et al. Multiportal endoscopic approaches to the central skull base: a cadaveric study. World Neurosurg. 73 (6), 705-712 (2010).
- Lan, Q., et al. Keyhole approach for clipping intracranial aneurysm: comparison of supraorbital and pterional keyhole approach. World Neurosurg. , (2017).
- Rodriguez-Hernandez, A., Walcott, B. P., Birk, H., Lawton, M. T. The Superior Cerebellar Artery Aneurysm: A Posterior Circulation Aneurysm with Favorable Microsurgical Outcomes. Neurosurgery. , (2017).
- Mazaris, P., et al. Endovascular Treatment of Complex Distal Posterior Cerebral Artery Aneurysms with the Pipeline Embolization Device. World Neurosurg. , (2017).
- Lee, S. H., et al. Surgical Flow Alteration for the Treatment of Intracranial Aneurysms That Are Unclippable, Untrappable, and Uncoilable. J Korean Neurosurg Soc. 58 (6), 518-527 (2015).
- Qian, Z. H., Feng, X., Li, Y., Tang, K. Virtual Reality Model of the Three-Dimensional Anatomy of the Cavernous Sinus Based on a Cadaveric Image and Dissection. J Craniofac Surg. , (2017).
- Shono, N., et al. Microsurgery Simulator of Cerebral Aneurysm Clipping with Interactive Cerebral Deformation Featuring a Virtual Arachnoid. Oper Neurosurg (Hagerstown). , (2017).
- Tomee, S. M., et al. The Consequences of Real Life Practice of Early Abdominal Aortic Aneurysm Repair: A Cost-Benefit Analysis. Eur J Vasc Endovasc Surg. , (2017).
- de Oliveira, M. M., et al. Learning brain aneurysm microsurgical skills in a human placenta model: predictive validity. J Neurosurg. , 1-7 (2017).
- Valentine, R., Padhye, V., Wormald, P. J. Simulation Training for Vascular Emergencies in Endoscopic Sinus and Skull Base Surgery. Otolaryngol Clin North Am. 49 (3), 877-887 (2016).
- Di Somma, A., et al. Extended endoscopic endonasal approaches for cerebral aneurysms: anatomical, virtual reality and morphometric study. Biomed Res Int. 2014, 703792 (2014).
- Oyama, K., et al. Endoscopic endonasal cranial base surgery simulation using an artificial cranial base model created by selective laser sintering. Neurosurg Rev. 38 (1), 171-178 (2015).
- Jukes, A. K., et al. Stress response and communication in surgeons undergoing training in endoscopic management of major vessel hemorrhage: a mixed methods study. Int Forum Allergy Rhinol. , (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved