Method Article
The Application of Remote-Controlled High-Definition Capsule Endoscopy in Canine Bladder Examination
In This Article
Summary
Here, we present a protocol for examining the canine bladder using a high-definition capsule endoscope, surgically implanted and maneuvered to capture images of the bladder wall and urination dynamics. The procedure offers insights for developing precise urodynamic studies.
Abstract
This study investigated the feasibility and potential applications of capsule endoscopy for bladder examination using a canine model. Three adult male Beagle dogs underwent surgical implantation of capsule cystoscopes and were divided into three groups, each group with one Beagle: Group A (Beagle A) remained in a fixed supine position for 8 h, Group B (Beagle B) moved freely for 8 h, and Group C (Beagle C) had manually adjusted positions (prone, supine, squatting) for 20 min each. The implanted capsule endoscopes functioned successfully in all dogs. Group A primarily captured images of the bladder base (6 h 27 min). Group B acquired images of the bladder neck and base (7 h 12 min), including dynamic visualization of the bladder neck, prostatic fossa, and external urethral sphincter during natural urination. Group C yielded images of the bladder neck, base, and apex (56 min). The findings of this study demonstrate the ability of capsule endoscopy to provide dynamic, high-quality images of the canine bladder wall and suggest its potential for developing accurate and urodynamic assessments.
Introduction
Transurethral cystoscopy, a common diagnostic tool in urological surgery, is widely used clinically not only as a reliable method for diagnosing bladder cancer but also as an important means for treatment and postoperative follow-up1. However, traditional cystoscopic examination, whether rigid or flexible, often causes discomfort to patients and may lead to complications such as urethral damage and retrograde infection2. Additionally, traditional urodynamic tests, by disturbing the normal physiological activity of the urinary tract and the mental and psychological activities of patients, inevitably introduce certain errors in the results3,4,5. Thus, the development of a miniaturized, comfortable, blind-spot-free, and more accurate diagnostic method represents a future direction for the advancement of cystoscopy.
Capsule endoscopy, also known as wireless endoscopy, has been extensively applied in gastrointestinal examinations, offering benefits such as convenience, painlessness, absence of cross-infection, and no disruption to normal patient activities. The painless acquisition of comprehensive gastrointestinal tract imaging data through capsule endoscopy has become a standard method6,7,8. Given that the bladder is a hollow organ connected to the external environment through the urethra, a capsule of appropriate size can be introduced into the bladder via the urethra.
Based on this, we propose the concept of a capsule cystoscope and explore its advantages and potential applications as a novel diagnostic tool through animal experiments, thereby providing new insights for the future development of capsule endoscopy technology. In this context, we hypothesize that the capsule cystoscope can obtain clear intravesical images and capture dynamic changes of different structures during the physiological urination process, providing insights for the development of more accurate urodynamic testing. This could potentially minimize patient discomfort in the future and expand the indications for cystoscopic examinations.
Protocol
This research was approved by the Medical Ethics Committee of the Affiliated Kunshan Hospital of Jiangsu University, strictly adhering to the Guidelines for the Ethics and Welfare of Experimental Animals. The ethical approval document number is 2021-06-008-K01.
1. Subjects
- Use three healthy adult male Beagles for the study. Randomly divide them into groups A, B, and C, with one dog per group.
- Inclusion criteria: Include animals that are 24 months old, approximately 12 kg in weight, and are free from cardiovascular, renal diseases, and other chronic conditions.
- Exclusion criteria: Exclude animals that have undergone any medicinal treatment received within a week before the experiment.
2. Experimental materials
- Conduct bladder examinations using a capsule endoscopy system, including a high-definition intelligent capsule, an image recorder, and a workstation. The capsule endoscope measures approximately 11 mm × 25 mm, captures images at 2 frames per second (FPS), operates for 8-10 h, and can transmit about 60,000 frames.
3. Experimental design
- Preoperative preparation
- Fast the Beagle dog for 8 h and withhold water for 2 h prior to surgery.
- Administer ampicillin (22 mg/kg) subcutaneously 30 min before surgery as a prophylactic antibiotic.
- Remove hair from the medial aspect of the left forelimb and the lower abdomen using clippers. Clean the surgical site with sterile saline and dry with sterile gauze. Disinfect the surgical site with povidone-iodine.
- Place a 20 G intravenous catheter in the cephalic vein of the left forelimb. Secure the Beagle dog to the operating table.
- Anesthesia
- Monitor vital signs. Administer dexmedetomidine hydrochloride (0.005 mL/kg) intravenously for sedation, followed by propofol (1.0 mL/kg) for induction of anesthesia.
- Insert an 8 mm endotracheal tube into the trachea using a laryngoscope. Confirm correct placement by auscultation and secure the tube with tape.
- Connect the Beagle dog to a veterinary anesthesia machine and maintain anesthesia with 1.5% to 3% isoflurane in oxygen via inhalation.
- Capsule endoscope implantation
- Place the Beagle dog in a supine position and position the image recorder nearby.
- Disinfect the lower abdomen three times with povidone-iodine and apply sterile surgical drapes.
- Make a 10 cm longitudinal incision adjacent to the penis using a #3 scalpel handle and a #10 blade. Incise the skin and subcutaneous tissues and bluntly dissect the abdominal muscles. Lift and retract the peritoneum using two hemostats.
- Locate the bladder and gently grasp it with atraumatic forceps. Lift and secure the bladder to prevent damage.
- Make a 1 cm incision on the bladder.
- Remove the capsule endoscope. Connect the image recorder to the workstation using the dedicated universal serial bus (USB) cable, then press and hold the power button on the image recorder for 3 s to power it on.
- Double-click the OMOM Ove icon on the workstation desktop. Enter the username and password to log in to the workstation software. Click Add Patient and enter the information for the Beagle, then click Save.
- In the Patient Information section of the workstation software, enter the capsule endoscope serial number and channel number to activate the capsule. Click Next, then click Create New Case. When prompted to format the device, select Yes.
- Check that the capsule endoscope is functioning properly. Click Real-time View to display the capsule endoscope images.
NOTE: In normal operation, the ACE indicator light on the image recorder and the LED on the capsule should blink synchronously. - Disinfect the capsule endoscope with povidone-iodine and place it into the bladder.
- Close the bladder incision with a continuous 4-0 absorbable suture. Close the abdominal wall layers, then close the skin with a 2-0 silk suture. Transfer the Beagle dog to a cage after surgery. During the transfer, keep the image recorder within 1 m of the Beagle dog to avoid disconnection from the capsule endoscope.
- Image acquisition
- Secure the image recorder to the top of the cage and ensure its safety.
- Click the Settings button on the workstation, then select Restart Image Recorder to reactivate the image recorder and confirm the capsule endoscope's operational status and connection.
- Confine Beagle A to a small dog cage for 8 h to maintain stillness. Allow Beagle B to move freely for 8 h.
- Have Beagle C maintain prone, supine, and squatting positions using gentle physical support and observe each position for 20 min, ensuring the animal's comfort and minimizing distress.
- Monitor and record bladder images from Beagle C using the image recorder. Click Real-time View to access the capsule's live video feed, then click the Start Recording button to initiate image acquisition and the Stop Recording button to terminate it. After sufficient data has been acquired, manually press the power button on the image recorder to turn it off.
- After approximately 8 h, when the ACT indicator light on the image recorders for Groups A and B has stopped flashing for 10 min, conclude the examination and turn off the recorders.
- At the end of the 8 h experimental period for Groups A and B, after image acquisition for Group C, anesthetize the dogs again following the procedures outlined in section 3.2.
- Surgically remove the capsule endoscope from the bladder through a cystotomy. Close the bladder and abdominal incisions as described in section 3.3.
Allow the dogs to recover in clean, quiet cages with soft bedding. Administer anti-infective and fluid therapy as needed. - Connect the image recorder to the workstation. Power on the image recorder and log in to the workstation software. Click Case Review; the system will then automatically download the image data. Save the image data to the workstation's hard drive once the download is complete.
- Analyze the acquired image data.
Results
In this study, each Beagle (n = 3) received one capsule endoscope surgically implanted into its bladder, and all animals demonstrated normal post-operative recovery. The capsule endoscopes functioned properly and remained safely within the dogs, as confirmed by imaging studies (Figure 2). Under remote control, the devices captured clear images of all anatomical regions of the bladder at various stages, including the dome, posterior wall, anterior wall, neck, and both the right and left side walls. In Beagle A, the endoscope acquired images for 6 h and 27 min, primarily of the bladder base (Figure 2A). In Beagle B, the endoscope obtained images for 7 h and 12 min, including images of the bladder neck in a resting state (Figure 2D), post-urination (Figure 2H), and the bladder base (Figure 2I). This group successfully captured the dynamic changes of the bladder neck-prostatic fossa-external urethral sphincter during the normal physiological urination process of the dogs. The key observations were: 1. The bladder neck opening at the onset of urination (Figure 2E). 2. Subsequently, the expansion of the prostatic fossa followed by the opening of the external urethral sphincter to allow urine flow (Figure 2F). 3. After urination, the external urethral sphincter closed first, followed by the prostatic fossa, and finally the bladder neck (Figure 2G), marking the end of urination (video of the physiological urination process collected is available as Supplementary Video 1). Beagle C was positioned in prone, supine, and squatting postures for 20 min each, totaling 56 min of image acquisition, including images of the bladder neck (Figure 2J), base (Figure 2K), and dome (Figure 2L).
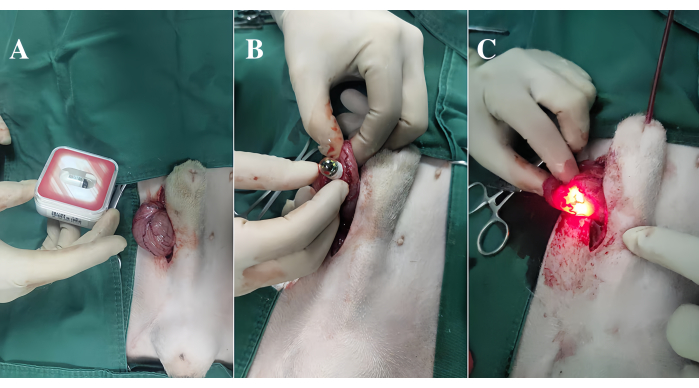
Figure 1: Process of inserting the endoscope into the bladder. (A) The incision and insertion of the capsule endoscope. (B) The activation of the capsule. (C) The suturing of the bladder Please click here to view a larger version of this figure.

Figure 2: The images obtained from capsule endoscopy and radiological localization of the capsule endoscope. (A) Image of the bladder base in Group A (purse-string suture bleeding). (B) Abdominal radiograph of a beagle in Group A showing the capsule located inside the bladder. (C) CT image of a beagle in Group A with the capsule inside the bladder. (D) Image of the bladder neck in Group B (resting state). (E) Image of the bladder neck of a beagle in Group B dilating, initiating urination. (F) Image of a beagle in Group B showing the bladder neck and prostatic fossa dilation, the external urethral sphincter opening, urine being expelled, and the capsule endoscope moving towards the bladder neck with the flow of urine. (G) Image of a beagle in Group B with the bladder neck closed, marking the completion of urination. (H) Image of the bladder neck post-urination in Group B. (I) Image of the bladder base in Group B. (J) Image of the bladder neck in Group C. (K) Image of the mid-bladder base in Group C. (L) Image of the bladder apex in Group C. Please click here to view a larger version of this figure.
Supplementary Video 1: Video of the physiological urination process acquired using the endoscope. Please click here to download this File.
Discussion
In recent years, with the advancement of endoscopic technology, both rigid and flexible cystoscopes have been widely applied in clinical practice. The conventional application of rigid cystoscopy is often cumbersome, with numerous blind spots and significant trauma. Patients experience high psychological stress during the procedure and may encounter discomfort or pain, as well as physiological responses such as increased heart rate, elevated blood pressure, and psychological stress reactions7. Flexible cystoscopy, on the other hand, offers more flexibility in operation, alleviates patient discomfort compared to rigid cystoscopy, and nearly eliminates blind spots. However, it still carries drawbacks, including the risk of cross-infection and susceptibility to damage9. Furthermore, traditional cystoscopic examinations lack the capability for prolonged dynamic observation of bladder mucosal status, relying heavily on the physician's individual experience, which may lead to misdiagnosis. Additionally, due to limited exploration time and restricted exploration angles, the diagnostic efficiency of traditional cystoscopy needs further improvement10,11. Therefore, there is an urgent clinical need for a convenient and sustainable examination method.
The implantation and retrieval of the capsule present significant challenges in this study. The capsule endoscopes currently widely used in clinical practice have a diameter exceeding 1cm. In our experiment, the capsule endoscope used has a diameter of 1.1 cm, which is wider than the narrowest part of a Beagle's urethra. Therefore, an open surgical procedure was adopted for the implantation of the capsule endoscope. The average diameter of the human urethra is 5-7 mm for males and 6 mm for females without dilation, expanding to 1 cm upon dilation12. The urethra exhibits a closing resistance, preventing the passage of currently available capsule endoscopes into the bladder. Consequently, the introduction and retrieval of a capsule endoscope through the urethra necessitate a smaller capsule size. It is noted that posterior urethral stones can be expelled from the bladder by hydrostatic pressure, and small stones can be passed through the urethra13. We infer that sufficiently small capsules could be introduced into the bladder via fluid or a catheter and expelled with urine. However, achieving such minimal sizes with current technology appears challenging. Given the small size required for capsule endoscopes, a lengthy battery life is unnecessary. This is supported by Group C findings, where most of the bladder images were rapidly acquired by changing the Beagle's body position. Thus, reducing the size of the battery might be a viable approach to decrease the overall size of capsule cystoscopes in the future. Wireless charging technology is also rapidly advancing across various sectors14. Furthermore, if the challenges of capsule size and battery life can be simultaneously addressed in the future, it would be possible to leave a capsule endoscope in the bladder for extended periods. This capability would allow for long-term monitoring of bladder malignancy recurrence and progression, potentially sparing patients from the discomfort of repeated cystoscopic examinations after bladder cancer surgery.
In this experiment, three groups, designated A, B, and C, obtained a total of 24 h of video data. Group A's footage was limited to views of the bladder's base; Group B's footage included views of both the bladder's base and neck, while Group C's footage encompassed views of the bladder's neck, base, and dome. This analysis was conducted for the following reasons. In standard capsule endoscopes, the camera end (front end) is heavier than the tail end, which contains air to ensure the camera end remains vertically aligned with the ground when in liquid. This was evidenced by Group A's footage, which consistently aligned with the bladder's base. Group B's experiment revealed that in the squatting position, the camera was aligned with the bladder's neck, and in the lying position, it was aligned with the bladder's base without capturing images of the bladder's dome. In Group C's experiment, by manually positioning the dogs in a supine position, images of the bladder's dome were successfully captured.
The comparison of the three experimental groups clearly indicates that when dogs are in their natural state, standard capsule endoscopes have blind spots in their observational range, making it difficult to capture complete images. By manually changing the dog's position, most of the bladder's inner wall can be captured. To address the limitation of capsule endoscopes' inability to move independently, current solutions include magnetic control systems and motor-driven propeller systems. The magnetic capsule was first designed and developed by Given Imaging and is now widely used in clinical settings, overcoming the traditional capsule endoscope's inability to capture complete images of the stomach15. The propeller-driven capsule was initially developed by the CRIM laboratory in 2009. It consists of three parts: a wireless controller, a battery, and a support shell containing four engines. Controlled wirelessly, the capsule can move at speeds of up to 7 cm/s in a fluid-filled stomach. Endoscopic capsules equipped with motion-assist systems have now been proven to move in a fluid-filled stomach and capture over 75% of image data16. Since the bladder lacks peristalsis and has a more stable internal environment compared to the stomach, this motion-assist system could also be applied to bladder capsules in the future to obtain more comprehensive imaging data of the bladder.
Beyond observing bladder abnormalities, cystoscopy importantly enables the retrieval of pathological materials using foreign body forceps. Currently, capsule endoscopes only possess observation capabilities, lacking the means to perform biopsies or therapeutic interventions. However, the future development of capsule endoscopy is progressively moving towards operational and therapeutic functionalities. In 2017, Son et al.17. introduced a novel magnetic actuation system for capsule endoscopy, which utilizes a thin, hollow needle within the capsule to puncture and aspirate tissue for sampling.
In this study, Beagle B underwent a successful capsule endoscopy, which captured the dynamic changes of the bladder neck, prostatic urethra, and external urethral sphincter during urination. At the onset of micturition, the bladder neck opened, followed by the expansion of the prostatic urethra, and finally, the external urethral sphincter opened. After urination concluded, the external urethral sphincter closed first, then the prostatic urethra, and finally the bladder neck. This represents the first observation of the dynamic changes in the bladder neck-prostatic urethra-external urethral sphincter complex in canines. We believe this holds significance for future urodynamic studies by introducing new approaches. Future developments could integrate pressure sensors into the capsule endoscope. During its expulsion through the urethra, dynamic imaging and pressure data from various sections of the bladder and urinary tract can be obtained. This reduces interference with the urinary tract and alleviates psychological stress for patients.
This study demonstrated the feasibility of using capsule endoscopy to capture images of the canine bladder, thereby laying the groundwork for its application in precise urodynamic evaluations. It highlights the potential for long-term dynamic monitoring in clinical settings through capsule cystoscopy. However, limitations include a small sample size and reliance on canine models, which may limit direct applicability to humans due to physiological differences in the urinary systems. Technical challenges currently facing capsule endoscopy in bladder examinations include miniaturizing the capsule, enhancing battery life, and refining control mechanisms. Furthermore, prolonged retention of the capsule in the bladder may impact urination dynamics, and extensive multi-angle observations could generate voluminous data. Nevertheless, ongoing advancements in technology and artificial intelligence are expected to overcome these hurdles, enhancing the precision and feasibility of capsule endoscopy for bladder assessments. Ultimately, this research not only reduces the disruption of normal urinary tract functions during examinations but also fosters the advancement of diagnostic technologies. This could revolutionize early detection and treatment strategies for bladder diseases, offering new avenues for medical innovation and patient care.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Funding: This work was supported by the Kunshan Science and Technology Development Special Project (KS18062), the Jiangsu University Clinical Science and Technology Development Project (JLY20180110), and the First People's Hospital of Kunshan's Scientific Education and Health Promotion Project (CXTD21-D02).
AUTHOR CONTRIBUTION:
Yang Yuan conceived the study, conducted experiments, and drafted the manuscript. Leyi Liu analyzed the data. Dingli Hu and Shihao Zhang provided critical resources and helped with data interpretation. Bing Wang contributed to literature review and manuscript editing. Yunlong Li, as the corresponding author, oversaw the project direction and manuscript finalization. All authors discussed the results and approved the final version of the manuscript for publication.
DATA AVAILABILITY:
All data generated or analyzed during this study are included in this article.
Materials
| Name | Company | Catalog Number | Comments |
| 2-0 Silk suture | Ethicon Inc. (Beijing) | 20193021851 | |
| 20 G intravenous catheter | Shanghai Zhangdong Medical Technology Co., Ltd. | 383012 | |
| 4-0 absorbable suture | Ethicon Inc. (Beijing) | 20193021851 | |
| 8 mm endotracheal tube | Henan Yadu Industrial Co., Ltd. | Not applicable | |
| Ampicillin | Chengdu Better Pharmaceutical Co., Ltd. | H19993625 | |
| Animal anesthesia machine | Nanjing Suprex Medical Equipment Co., Ltd. | Not applicable | |
| Animal ECG monitor | Smiths Medical (US) | Not applicable | |
| Animal laryngoscope | Shanghai Maiben Medical Technology Co., Ltd. | Not applicable | |
| Beagle | School of Agriculture and Biology, Shanghai Jiao Tong University | Not applicable | |
| Gauze Sponges | Gauze Sponges | 13-761-52 | |
| Isoflurane | Abbott Laboratories (Shanghai) | H20059911 | |
| OMOM intelligent capsule endoscope | Chongqing Jinshan Science & Technology (Group) Co., Ltd. | NCG100 | |
| Povidone-iodine solution | Chengdu Yongan Pharmaceutical Co., Ltd. | H51022885 | |
| Propofol injection | Xi'an Libang Pharmaceutical Co., Ltd. | H19990281 | |
| Scalpel blade | Shanghai Pudong Golden Ring Medical Supplies Co., Ltd. | 35Y1004 | |
| Sterile normal saline | Shijiazhuang No.4 Pharmaceutical Factory | H20066533 | |
| Surgical instruments | Johnson & Johnson Medical (Shanghai) | Not applicable | |
| Dexmedetomidine hydrochloride | Jiangsu Hengrui Medicine Co., Ltd. | H20190407 |
References
- Matulewicz, R. S., Delancey, J. O., Meeks, J. J. Cystoscopy. JAMA. 317 (11), 1187(2017).
- Von Rundstedt, F. C., Lerner, S. P. New imaging techniques for nonmuscle invasive bladder cancer. Curr Opin Urol. 24 (5), 532-539 (2014).
- Chan, G., Qu, L. G., Gani, J. Evaluation of pre-operative bladder contractility as a predictor of improved response rate to a staged trial of sacral neuromodulation in patients with detrusor underactivity. World J Urol. 39 (6), 2113-2119 (2021).
- Stav, K., Siegel, Y. I., Beberashvili, I., Sella, H. Z., Zisman, A. Provision of information leaflet before urodynamic study reduces the pre-examination anxiety level. Neurourol Urodyn. 35 (7), 805-808 (2016).
- Vogt, B. Catheter-free urodynamics testing: Current insights and clinical potential. Res Rep Urol. 16, 1-17 (2024).
- Akpunonu, B., Hummell, J., Akpunonu, J. D., Ud Din, S. Capsule endoscopy in gastrointestinal disease: Evaluation, diagnosis, and treatment. Cleve Clin J Med. 89 (4), 200-211 (2022).
- Alkhamees, M., et al. Reusable vs. Single-use cystoscope for removal of double-j stent: A prospective randomized comparison and cost analysis. Eur Rev Med Pharmacol Sci. 26 (12), 4268-4273 (2022).
- Vuik, F. E. R., et al. Colon capsule endoscopy in colorectal cancer screening: A systematic review. Endoscopy. 53 (8), 815-824 (2021).
- Lee, J., Kaplan-Marans, E., Jivanji, D., Tennenbaum, D., Schulman, A. Post-cystoscopy infections and device malfunctions in reprocessed flexible cystoscopes in a national database. Can J Urol. 29 (6), 11361-11365 (2022).
- Ikeda, A., et al. Support system of cystoscopic diagnosis for bladder cancer based on artificial intelligence. J Endourol. 34 (3), 352-358 (2020).
- Chan, E. O., Pradere, B., Teoh, J. Y. The use of artificial intelligence for the diagnosis of bladder cancer: A review and perspectives. Curr Opin Urol. 31 (4), 397-403 (2021).
- Wessells, H., Morey, A., Souter, L., Rahimi, L., Vanni, A. Urethral stricture disease guideline amendment (2023). J Urol. 210 (1), 64-71 (2023).
- He, M., et al. Recent advances in the treatment of renal stones using flexible ureteroscopys. Int J Surg. 110 (7), 4320-4328 (2024).
- Gao, Z., et al. Advanced energy harvesters and energy storage for powering wearable and implantable medical devices. Adv Mater. 36 (42), e2404492(2024).
- Swain, P., et al. Remote magnetic manipulation of a wireless capsule endoscope in the esophagus and stomach of humans (with videos). Gastrointest Endosc. 71 (7), 1290-1293 (2010).
- Tortora, G., et al. Propeller-based wireless device for active capsular endoscopy in the gastric district. Minim Invasive Ther Allied Technol. 18 (5), 280-290 (2009).
- Son, D., Gilbert, H., Sitti, M. Magnetically actuated soft capsule endoscope for fine-needle biopsy. Soft Robot. 7 (1), 10-21 (2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved