Method Article
A Simple Cuff Technique for Murine Left Lung Transplantation
In This Article
Summary
The present protocol describes a cuff technique for a mouse left lung transplantation model. This technique has been developed over several years and has performed well, serving effectively in immunological research.
Abstract
Over the past decade, our laboratory has made significant progress in developing and refining vascularized mouse lung transplantation models using an efficient and highly reliable "cuff technique" of transplantation. This article describes a sophisticated and comprehensive method for orthotopic lung transplantation in a vascularized orthotopic lung model, representing the most physiologic and clinically relevant model of mouse lung transplantation to date. The transplantation process consists of two distinct stages: donor harvest and subsequent implantation into the recipient. The method has been successfully mastered, and with several months of sufficient training, a skilled practitioner can perform the procedure in approximately 90 min from skin-to-skin. Surprisingly, once individuals overcome the initial learning curve, the survival rate during the perioperative period approaches nearly 100%. The mouse model allows for the use of multiple commercially available transgenic and mutant strains of mice, enabling the study of tolerance and rejection. Additionally, the unique features of this model make it a valuable tool for investigating tumor biology and immunology.
Introduction
While replacement therapies, such as dialysis and ventricular assist devices, exist for those with renal and heart failure, lung transplantation remains the primary treatment option for patients suffering from end-stage pulmonary disease. This procedure serves as the sole life-saving choice for individuals diagnosed with pulmonary fibrosis1. Additionally, it is employed to extend the lifespan of those facing end-stage obstructive lung diseases like emphysema, as well as those with suppurative conditions such as cystic fibrosis1.
While short-term survival rates have improved due to technical refinements, perioperative care enhancements, and advances in immunosuppression, long-term outcomes after lung transplantation are markedly inferior to those of other solid organs. The overall five-year survival rate of only 50% for lung graft recipients is much lower than for those receiving heart, kidney, or liver allografts1. Our team and others have suspected that the reason for such a discrepancy is the lack of clinically relevant biological models to fully understand pathways leading to lung allograft rejection and/or tolerance, as experimental manipulation of physiologically relevant mouse models has significantly contributed to the long-term survival of other solid or cellular allografts2.
Prior to the development of the mouse orthotopic lung transplant model, researchers relied on larger animal models for their studies. However, these larger models had limitations, including a lack of transgenic mutants required for exploring mechanistic questions3. Additionally, rat studies faced similar limitations4, and non-vascularized heterotopic tracheal transplant models in mice were also limited to their utility5. Research has shown the importance of using vascularized transplant models instead of nonvascularized ones in various organ systems. For example, nonvascularized grafts of neonatal cardiac tissue inserted into the ear pinna of recipient mice lack direct interaction with vascular endothelium and recipient bloodstream. Therefore, they are limited in their relevance compared to vascularized human cardiac grafts6. In a similar manner, the heterotopic tracheal transplant model, which lacks vascularization and aeration, differs significantly from human lung transplants, particularly in the observed airway changes in small airways after transplantation. Moreover, the rapid onset of fibrotic occlusion in heterotopic tracheal allografts does not mirror observations in human lungs or in vascularized lung grafts in larger animals or rats7.
In our research lab, the creation of the orthotopic mouse lung transplant model was inspired by the orthotopic single lung transplant model established in rats8,9. Unlike humans, mice, and rats possess only one lobe in their left lung, constituting a mere twenty-five percent of the total lung mass. This feature enables the successful execution of left lung transplantation in murine models without requiring circulatory support10,11. Over time, we have introduced technical modifications to the model, and in this context, the key steps that enhance the ease of performing the procedure are elucidated.
Beyond its implications in transplant immunobiology research, the orthotopic mouse lung transplant model offers a robust instrument for exploring the role of pulmonary non-hematopoietic stromal cells in various disease processes. This occurs because a mutant syngeneic single lung transplant leads to an almost complete replacement of hematopoietic cells with recipient-derived ones, whereas non-hematopoietic stromal cells continue to originate from the donor. Consequently, a "chimeric organ" can be created without host irradiation or lung-specific expression of a transgene12. Moreover, the opposite-side native lung can function as an internal control within the same animal without causing widespread changes to the host's immune system. This model holds great promise for advancing research in lung transplantation and other disease pathways involving pulmonary stromal cells.
Protocol
All animal-related procedures were conducted in accordance with and received approval from the Institutional Animal Care and Use Committee at The University of Maryland, Baltimore. It is recommended to use male, 8-12 weeks (20-25 g), BALB/c mice as the donor and C57BL/6 mice as the recipient. The animals were obtained from a commercial source (see Table of Materials).
1. Preparation of bronchial and vascular cuffs
Timing: 10 min (for three cuffs)
- Prepare the bronchial cuff using an 18 G angiocatheter and the pulmonary venous cuff using a 20 G angiocatheter having a length of 1 mm (excluding the extension handle) (see Table of Materials). Utilize a #11 scalpel and the metal needle of the angiocatheter serving as the cutting surface (Figure 1).
- Prepare the pulmonary artery cuff using a 24 G angiocatheter having a length of 0.6 mm (see Table of Materials) in a similar manner.
NOTE: General fundamental principles of the cuff technique for creating microvascular anastomoses is as follows: when establishing microvascular anastomoses for mouse lung transplantation, specific cuff techniques are utilized due to the small size of the vessels. In contrast to larger vessels, which can be directly sutured, the microscopic bronchial and vascular connections necessitate a distinct method. The fundamental principle involves passing the donor vessel through a rigid circular cuff that is slightly larger than the vessel itself.
2. Donor procedure
Timing: 10-15 min
- Administer intraperitoneal (i.p.) injection of 50 mg/kg ketamine and 10 mg/kg xylazine (see Table of Materials) to anesthetize the donor mouse (following institutionally approved protocols).
- After ensuring adequate anesthesia via toe-pinch, shave the donor mouse and place in a supine position under the operating microscope at 10x magnification.
- Inject 50 uL of heparin (see Table of Materials) through the penile vein (Figure 2A).
- Cut down on trachea and insert a 20 G angiocatheter and then encircle it with a 6-0 silk suture (see Table of Materials). Connect the catheter to the ventilator (Figure 2B).
- Perform a midline abdominal incision (laparotomy) and make a circumferential incision in the diaphragm (Figure 2C).
- Conduct a midline sternotomy12 and position two forceps on either side of the sternum for hemostasis and retraction from the bone edges (Figure 2D).
- Remove the thymus and excise the right and left atrial appendages to facilitate the venting of any drainage from the lungs.
- Inject 3 mL of cold 4 °C commercially available preservation solution (see Table of Materials) directly into the right ventricle or root of pulmonary artery, using a 27 G needle on a 3 mL syringe in order to flush all the blood from the lungs.
NOTE: Intraventricular or pulmonary artery injection may cause injury to the donor lung. It is crucial to maintain adequate ventilation at the time of flushing to ensure an even distribution of the preservation solution. - Clamp the trachea proximally and then divide the trachea distally. Next, dissect the trachea caudally and posteriorly12.
- Remove the heart-lung block and transfer it to a small Petri dish, sterile and lined with gauze moistened with a cold preservation solution.Then, put this Petri dish into a larger one filled with ice (Figure 2E).
- To expose left hilum, place small gauze on both left and right lung tissues for retraction (Figure 2F).
- Gently separate the hilar structures from one another using curved forceps with precision. Dissect the left pulmonary artery (PA) from the bronchus (Figure 2G). Next dissect the pulmonary vein (PV), located in the most caudal aspect of the hilum from the bronchus in a similar fashion (Figure 2H).
- Cut the PA and PV at the longest length possible for proper cuffing. Try to avoid excising excessive fat or lymphoid tissue, since this could make the cuffing step hard to perform.
- Position the PV cuff approximately 2 mm above the pulmonary artery using the stabilization clamp, and then insert the PV inside the cuff. Fold the PV over the cuff, thereby exposing the endothelial surface (Figure 2I). Secure it around the cuff with 10-0 suture (2 knots) (Figure 2J).
- Divide PA away from hilum and place cuff on PA in an identical fashion.
NOTE: Refrain from cutting the bronchus at this stage. Instead, make sure to cuff the bronchus just before implantation in order to prevent the entry of the preservation solution into the airway at the time of storage. Submerge the lung block in the preservation solution at a temperature of 4 °C.
3. Recipient procedure
Timing: 50-60 min
- Anesthetize the recipient mouse with a mixture of ketamine (50 mg/kg) and xylazine (10 mg/kg) (intraperitoneally) (following institutionally approved protocols). Next, shave the left chest and intubate12.
- Conduct the intubation under direct vision by retracting gently the tongue out of the mouth and thereby exposing the vocal cords. Perform this under the dissecting scope with 10x magnification. Intubate the animal by inserting a 20 G x 1.25" angiocatheter directly between the vocal cords.
- Place the animal in right lateral decubitus position and clean chest wall with 70% ethanol. (Figure 3A).
- Use scissors to incise the skin and complete the left thoracotomy entirely with electrocauter (see Table of Materials).
NOTE: To minimize blood loss from the chest wall during this step, utilize electrocautery for all portions of this dissection. The skin must be dried after wiping with ethanol, as electrocautery carries a burn risk when combined with ethanol. - Enter the chest from 3rd or 4th intercostal space and place two chest retractors (see Table of Materials) (Figure 3B).
- After the thoracotomy, transition the mouse to a supine position. Slightly bend the recipient mouse's body to bring the left chest closer to the operator, enhancing the view of the hilum for improved visibility and accessibility.
- Position a right angle artery forceps (6 inches) on the recipient's left lung and gently retract the lung. It allows for the exposure of the anterior hilum, facilitating further surgical access and manipulation (Figure 3C).
- Dissect the pulmonary artery (PA) from the bronchus using curved dissection forceps, ensuring not to tear the vessel. The optimal dissection location is the middle part, and a distance of 2 mm is sufficient (Figure 3D).
- Dissect PV from bronchus in a similar fashion (Figure 3E).
- To obstruct the recipient's left lung airflow and blood flow, place an aneurysm microclip (see Table of Materials) at base of the hilum (Figure 3F).
- Make a small V-shaped incision at the recipient's pulmonary artery (PA) and pulmonary vein (PV) using microscissors. Gently dilate the bronchial and vascular openings using straight or curved forceps to generate an appropriate opening for placing the donor cuffs.
- Gently flush with diluted heparin (heparin: saline = 1:9) to remove blood.
- Place 10-0 nylon around the PA and leave it loose (for securing the cuffs at a later stage).
- Then, cut the donor bronchus and cuff it in a similar fashion to the PA and PV. Discard the donor right lung-heart block.
- Holding the recipient left lung, position the donor lung on top of retraction forceps and cover it with a small moist gauze.
NOTE: The recipient's left lung should be kept in position to apply tension to the recipient hilum, thereby facilitating the anastomosis process. - Anteriorly expose the cuffed hilar structures and start the anastomoses by placing the donor into the recipient pulmonary artery (PA) while inferiorly holding the cuff. At this point the donor's PA will experience some stretch (Figure 3G).
- Once the donor pulmonary artery (PA) is positioned into the recipient PA, secure the structures by placing a microclip across the cuff extension. Next, secure the previously placed 10-0 nylon suture around the recipient pulmonary artery (PA) and cuff with two knots (refer to Figure 3H). Finally, detatch the microclip from the extension cuff.
- Insert the donor's PV into the recipient's PV in a similar manner (Figure 3I).
- Insert the donor bronchus into the recipient bronchus, ensuring that the donor bronchus is under some stretch (Figure 3J). Release the microclip on hilum to restore perfusion and ventilation (Figure 3K).
- In the chest cavity, insert the donor lung and excise the recipient left lung by cutting the remaining pieces of the recipient pulmonary artery (PA), pulmonary vein (PV), and bronchus anteriorly (Figure 3L). Dispose of the recipient left lung.
- Close the chest using 6-0 PDS suture (see Table of Materials), thereby securing one rib below and one rib above the thoracotomy incision. Just before closing the chest, provide the animal with one large breath to expel any remaining air from the chest.
NOTE: Unlike in the rat model, there is no need to insert chest tubes into mice. - Suture the skin and the underlying subcutaneous tissue using a continuous subcuticular 6-0 silk suture (see Table of Materials).
4. Animal recovery
Timing: 12 h
- Allow the animal to recover with unrestricted access to food and water in the small animal ICU (portable animal intensive care unit, see Table of Materials) overnight.
- Administer Buprenorphine subqutaneously (0.5-1 mg/kg) every 12 h to minimize post-operative pain.
Results
Based on the experience with this model over the last 10 years, individuals with basic microsurgical skills typically require a learning curve of approximately 50 animals. Once proficiency is achieved, the donor procedures typically take 15-30 minutes, while the recipient procedures take approximately 60 min. After the initial learning curve, perioperative mortality tends to be very low.
In Figure 4A,B, the lung morphology seven days following the engraftment of Balb/cJ donor left lungs into C57/B6J recipients shows that mice treated with co-stimulatory blockade exhibited preserved, normal morphology. In contrast, non-immunosuppressed mice displayed signs of rejection.
In Figure 4C,D, the H & E staining of co-stimulatory blockade-treated mice revealed minimal immunoreactivity, suggesting immune tolerance. In contrast, non-immunosuppressed mice exhibited prominent lymphocytic infiltration, indicative of a rejection response.

Figure 1: Cuff creation. Employing blade #11, (A) vascular and bronchial cuffs are made with an extension cuff handle to ease the placement. (B) 18 G/20 G/24 G catheters were used for bronchos/pulmonary vein/pulmonary artery (from left to right). Please click here to view a larger version of this figure.
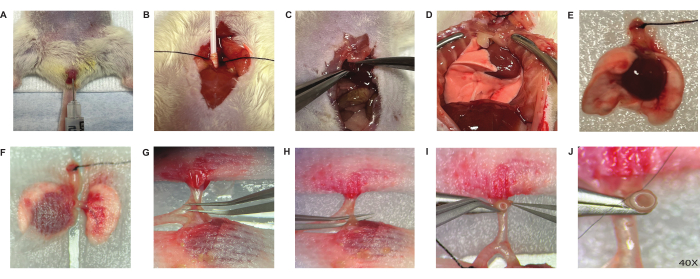
Figure 2: Donor operation. The donor procedure proceeds with heparin injection through the penis vein (A), intubation (B), dissection of the diaphragm (C) and a midline sternotomy (D). The excised lung block (E) was kept moist with guaze pads covered (F). The hilum is dissected starting with the donor plumonary artery (G), pulmonary vein (H). The vessel was grabbed through the lumen of the cuff and then folded over the cuff exposing the endothelial/epithelial surface(I). Next, it was fastened with 10-0 nylon suture around the cuff (2 knots) (J). Please click here to view a larger version of this figure.
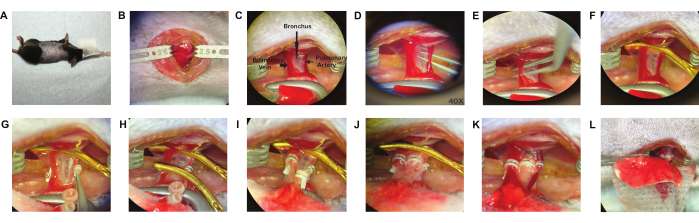
Figure 3: Recipient operation. The recipient procedure proceeds with endotracheal intubation, right lateral decubitus position (A) and a left thoracotomy (B). The recipient left lung is released from the attachments in the hilum, anterior view (C) and the recipient pulmonary artery (D), pulmonary vein (E) and bronchus are dissected. Proximal control of the recipient hilum is obtained with an aneurysm clip (F). The cuffed donor pulmonary artery is inserted into the recipient pulmonary artery through a V-shaped 1/3 arteriotomy (G) and the cuff handle is controlled by a second aneurysm clip to keep cuff stable, then secured with a 10-0 nylon tie (2 knots) (H). The 2nd clip was removed and the same procedure was followed for the pulmonary vein (I) and bronchus (J). The 1st clip was removed to let the lung reperfuse (K). the lung is inflated and then placed back in the chest (L). Please click here to view a larger version of this figure.
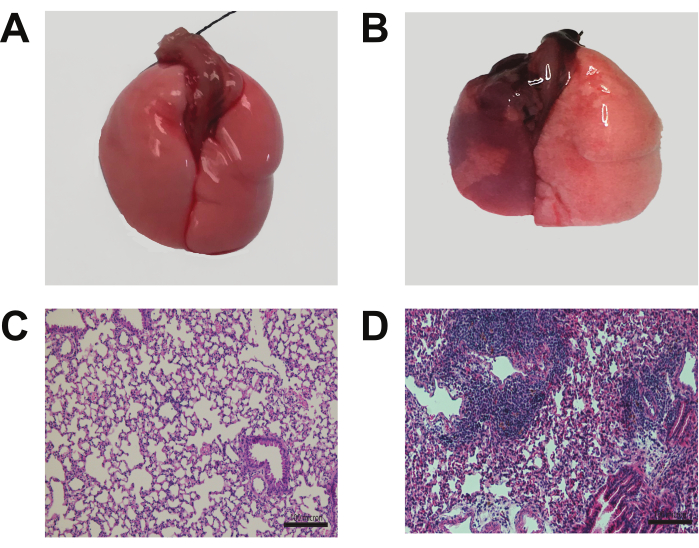
Figure 4: Morphology and histology of the transplanted lung. Seven days following engraftment of Balb/cJ donor left lungs into C57/B6J recipients, mice treated with co-stimulatory blockade exhibited a preserved, normal morphology (A). In contrast, non-immunosuppression treated mice displayed signs of rejection (B). H & E staining of the co-stimulatory blockade-treated mice revealed minimal immunoreactivity, suggesting immune tolerance (C). Non-immunosuppression treated mice exhibited prominent lymphocytic infiltration, indicative of a rejection response (D). Scale bars: 100 µm. Please click here to view a larger version of this figure.
Discussion
The cuff technique for murine left lung transplantation represents a significant advancement in transplantation research10,11. Critical steps include precise and meticulous hilar structure dissection and secure anastomoses. Modifications can be made to suit experimental needs, but a learning curve is involved. Our group has modified the recipient mouse position from posterior to anterior, gaining a better hilum view by bending the recipient mouse, which shortens the learning curve.
Limitations include challenges with mice outside the specified weight range and the need for skilled surgeons with prior experience in microsurgery. To mitigate the risk of graft atelectasis, it is strongly advised to implement positive end-expiratory pressure (PEEP) after engraftment and ensuring the appropriate administration of analgesics. For the prevention of venous tears, it is recommended employing a smaller cuff size (22 G). Additionally, to avert arterial torsion, it is suggested to minimize the graft movement after preparation and taking utmost care when adjusting the orientation of the donor's lung during the anastomosis procedure.
Despite limitations, the method's vascularized approach surpasses nonvascularized models and provides better physiological relevance. It also overcomes shortcomings of heterotopic tracheal models7, making it an ideal model for the study of transplant immunobiology and disease processes. The model's transformative feature allows the gradual replacement of donor hematopoietic cells by host cells, facilitating research on disease and malignancy12. Additionally, it offers insights into pulmonary stromal cell contributions, essential for understanding lung pathophysiology and targeted therapies.
Overall, the cuff technique holds promise for advancing research in transplantation biology, lung diseases, and immunology, with potential applications in diverse fields. Researchers should be mindful of its learning curve and adaptability to specific research needs. We aim to improve the efficiency and success of the procedure by making practical modifications, such as repositioning the animal after thoracotomy for a better surgical view. While the core technique remains the same, these enhancements can significantly ease the process, especially for timely lung anastomosis in experimental settings. This article provides valuable tips for better technique performance in the field.
Disclosures
The authors have nothing to disclose relevant to the subject of this manuscript.
Acknowledgements
ASK, AEG and DK are supported by P01 AI116501. ASK and EJ are further supported by R01AI145108-01, R01HL166402. ASK is supported by I01 BX002299-05. AEG and DK are further supported by RO1HL09601. CL is supported by R01 HL128492. This work is partially supported by Chuck and Mary Meyers and Richard and Eibhlin Henggeler.
Materials
| Name | Company | Catalog Number | Comments |
| 10-0 Nylon suture | Surgical Specialties Corporation, Reading PA | AK-0106 | |
| 2 Dumont #5 forceps | Fine Science Tools Inc., Foster City, CA | 11251-20 | |
| 2 Halsted-Mosquito clamp curved tip | Fine Science Tools Inc., Foster City, CA | 91309-12 | |
| 6-0 braided silk suture | Henry Schein Inc., Melville, NY, | 100-5597 | |
| 6-0 Polydioxanone PDS II suture and | Ethicon Inc., Somerville, NJ. | Z117H | |
| 70% Ethanol | Pharmco Products Inc., Brookfield, CT | 111000140 | |
| Adson forceps | Fine Science Tools Inc., Foster City, CA | 91127-12 | |
| Balb/c mice | Jackson Laboratories, Bar Harbor, Maine, USA | 000651 | 8–12 weeks; Male |
| Bipolar coagulator | Valleylab Inc., Boulder, CO | SurgII-20, E6008/E6008B | |
| C57BL/6 mice | Jackson Laboratories, Bar Harbor, Maine, USA | 000664 | 8–12 weeks; Male |
| Clear chlorhexidine | Hibiclens, Mölnlycke Health Care US, LLC, Norcross, GA | 57591 | |
| Electrocautery | Bovie | ||
| Fine vannas style spring scissors | Fine Science Tools Inc., Foster City, CA | 15000-03 | |
| Halsey needle holder | Fine Science Tools Inc., Foster City, CA | 91201-13 | |
| Harvard Apparatus Mouse Ventilator VentElite | Harvard Apparatus, Holliston, MA | 55-7040 | settings 3L O2/minute, respiratory rate 130 bpm, 0.4 cc tidal volume |
| Heparin solution | Abraxis Pharmaceutical Products, Schaumburg, IL | 504031 | 100 U/mL |
| Injection grade normal saline | Hospira Inc., Lake Forest, IL | NDC 0409-4888-20 | |
| Ketamine | VetOne, Boise, ID | 501072 | 50 mg/kg |
| Konig Mixter Micro Pediatric Forceps Right-Angled Jaws | Medline, Northfield, IL, | MDS1247714 | Extra Fine, Overall Length 5 1/2" (14cm) |
| Medline High Temperature Cautery,W/ Fine Tip | Leica Microsystems, Inc., Allendale, NJ | 10 450 290 | |
| Microscope Leica M80 F12 Floor Stand | Fine Science Tools Inc., Foster City, CA | 15396-00 | |
| Moria extra fine spring scissors | Parkland Scientific, Coral Springs, FL | V3000i | |
| Ohio Isoflurane Vaporizer | Vitrolife Inc., Englewood, CO, | 19001 | |
| Perfadex low-potassium dextran glucose solution | Becton Dickinson Labware, Franklin Lakes, NJ | 353025 | Electrolyte preservation solution |
| polystyrene petridishes | Fine Science Tools Inc., Foster City, CA | 00632-11 and 00649-11 | 150 × 25 mm and 60 × 25 mm |
| S&T SuperGrip Forceps straight and angled tip | Fine Science Tools Inc., Foster City, CA | 18200-20 | |
| Small animal retraction system | Puritan Medical Company LLC, Guilford, Maine | 823-WC | tapered mini cotton tipped 3 inch applicators |
| sterile Q-tips | Terumo Medical Corporation, Elkton, MD | SROX2419Z | |
| Surflo etfe IV Catheter, Yellow, 24 G x 0.75" | Terumo Medical Corporation, Elkton, MD | SROX1851Z | |
| Surflo etfe IV Catheter; Green, 18 G x 2" | Terumo Medical Corporation, Elkton, MD | SROX2032Z | |
| Surflo etfe IV Catheter; Pink, 20 G x 1.25" | Thermocare, Inc., Incline Village, NV | ||
| ThermoCare Small Animal ICU System, | A to Z Vet Supply, Dresden, TN | 008679 | 10 mg/kg |
| Xylazine | Aesculap, Inc., Center Valley, PA, | FT480T | |
| Yasargil Clip Applier | Aesculap, Inc., Center Valley, PA, | FT264T | |
| Yasargil Temporary Aneurysm Clips | Medline, Northfield, IL, | ESCT001 |
References
- Trulock, E. P., et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult lung and heart-lung transplantation report-2007. J Heart Lung Transplant. 26 (8), 782-795 (2007).
- Truong, W., Emamaullee, J. A., Merani, S., Anderson, C. C., James Shapiro, A. M. Human islet function is not impaired by the sphingosine-1-phosphate receptor modulator FTY720. Am J Transplant. 7 (8), 2031-2038 (2007).
- Ziegler, A., Gonzalez, L., Blikslager, A. Large animal models: The key to translational discovery in digestive disease research. Cell Mol Gastroenterol Hepatol. 2 (6), 716-724 (2016).
- Lin, J. H. Applications and limitations of genetically modified mouse models in drug discovery and development. Curr Drug Metab. 9 (5), 419-438 (2008).
- Hele, D. J., Yacoub, M. H., Belvisi, M. G. The heterotopic tracheal allograft as an animal model of obliterative bronchiolitis. Respir Res. 2 (3), 169-183 (2001).
- Djamali, A., Odorico, J. S. Fas-mediated cytotoxicity is not required for rejection of murine nonvascularized heterotopic cardiac allografts. Transplantation. 66 (12), 1793-1801 (1998).
- Neuringer, I. P., et al. Epithelial kinetics in mouse heterotopic tracheal allografts. Am J Transplant. 2 (5), 410-419 (2002).
- Okazaki, M., et al. Sphingosine 1-phosphate inhibits ischemia reperfusion injury following experimental lung transplantation. Am J Transplant. 7 (4), 751-758 (2007).
- Mizuta, T., Kawaguchi, A., Nakahara, K., Kawashima, Y. Simplified rat lung transplantation using a cuff technique. J Thorac Cardiovasc Surg. 97 (4), 578-581 (1989).
- Okazaki, M., et al. A mouse model of orthotopic vascularized aerated lung transplantation. Am J Transplant. 7 (6), 1672-1679 (2007).
- Gelman, A. E., et al. CD4+ T lymphocytes are not necessary for the acute rejection of vascularized mouse lung transplants. J Immunol. 180 (7), 4754-4762 (2008).
- Krupnick, A. S., et al. Orthotopic mouse lung transplantation as experimental methodology to study transplant and tumor biology. Nat Protoc. 4 (1), 86-93 (2009).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved