Method Article
Establishment of a Robust and Reproducible Model of Radiation-Induced Skin and Muscle Fibrosis
* These authors contributed equally
In This Article
Summary
Here we present a protocol to induce radiation-induced skin fibrosis in the hind limb of mice and perform post-irradiation measurements of chronic impairment via limb excursion and gait index analyses to evaluate the functional outcome. The model elucidates radiation-related skin fibrosis mechanisms and is useful in subclinical therapeutic studies.
Abstract
Radiation-induced skin fibrosis (RISF) can result from a plethora of scenarios including cancer therapy, accidental exposure, or acts of terrorism. Radioactive beams can penetrate through the skin and affect the structures in their path including skin, muscles, and internal organs. Skin is the first structure to get exposed to radiation and is susceptible to develop chronic fibrosis, which is challenging to treat. Currently, limited treatment options show moderate efficacy in mitigating radiation-related skin fibrosis. A key factor hindering the development of effective countermeasures is the absence of a convenient and robust model that could allow for translation of the experimental findings to humans. Here, a robust and reproducible murine hind limb skin fibrosis model has been established for prophylactic and therapeutic evaluation of possible agents for functional and molecular recovery.
The right hind limb was irradiated using a single dose of 40 (Gray) Gy to induce skin fibrosis. Subjects developed edema and dermatitis in the early stages proceeded by visible skin constriction. Irradiated limbs showed a significantly reduced limb range of motion in the following weeks. In late stages, acute side effects subsided, yet chronic fibrosis persisted. A gait index was performed as an additional functional assay, which demonstrated the development of functional impairment. These non-invasive methods demonstrated reliable measurements for tracing fibrosis progression, which is supported by histological analyses. The radiation dose, application, and post-irradiation analyses employed in this model offer a vigorous and reproducible method for studying radiation-induced skin fibrosis and testing the efficacy of therapeutical agents.
Introduction
The skin is the largest organ of the body, covering and protecting the body from hazards. It has three distinct layers: epidermis, dermis, and hypodermis. Each layer has its unique functions: the epidermis prevents dehydration and microbial invasion; the dermis has a rich network of cells, and an extracellular matrix that provides tensile strength and elasticity1; the dermal layer contains the sensory receptors, hair follicles, glands, and vessels for lymphatic and capillary networks. The hypodermis or subcutaneous tissue, with its abundance of adipose tissue, contours the body and distributes mechanical stress2,3,4.
Radiation, generated as a result of accidents, war, terrorism, or therapeutical applications, penetrate through the body in a linear progressive nature, leading the skin to be the first organ to come in contact. The threat of such incidents has intensified due to the increased use of radioactive materials in industries, medical facilities, and military installations5. Clinically, radiation damage to the skin is characterized by cutaneous radiation syndrome (CRS), one of four sub-syndromes of acute radiation syndrome (ARS). The response of the skin to ionizing radiation has important implications for treatment and protection from further damage6. Concomitant injuries such as burns and trauma further complicate the clinical outcome when combined with radiation injuries7. The extent of skin exposure to radiation correlates to a point-of-no-return threshold, from which the impairment of other organs results in single or multiple organ failure, and ultimately leads to patient death8,9. Cutaneous radiation injury is comprised of an acute and a chronic phase. Acute radiation injury clinically manifests as erythema, skin edema, dermatitis, blistering, epidermal denudation, dry or wet desquamation, ulceration, and changes in the hair and nails. The chronic phase is manifested as dermal atrophy, fibrosis, chronic ulceration, and telangiectasias10,11. In general, acute effects are predominately manifested in the epidermis, while chronic effects are most prominent in the dermis. Acute reaction to radiation exposure leads to a marked decrease in mitotic activity within 12 h of exposure, followed by hyperemia, cell enlargement, vacuolization, nuclear pyknosis, and fragmentation4,12.
Radiation doses exceeding 40 Gy result in moist desquamation and loss of epidermis, leading to an increased susceptibility to infections13. In addition, skin exposure to radiation induces cytokine production, triggering an inflammatory immune response in the dermal layer. Prominent inflammatory mediators include interleukins (IL-1, IL-3, IL-5, IL-6, and IL-8) and tumor necrosis factor-α (TNFα)14. Failure in the resolution of inflammation can eventually result in fibrosis development at the site of radiation injury15. Additional physical wounds or thermal injuries further aggravate this fibrotic response, extending through the muscle layer16. Transforming growth factor-β (TGFβ) is the key cytokine in fibrosis development17. Currently, very few treatment options show promising results, and the majority might have challenges with patient compliance. Further research exploring the cellular and molecular responses of the skin to different radiation doses will improve the understanding of the radiation-induced skin pathophysiology and enhance the development of new therapies.
To facilitate the clinical translation of research outcomes in preclinical models in alleviating radiation-induced injury to the skin and soft tissues, designing highly relevant experimental models of therapeutic interventions following irradiation is crucial. Both in vitro and in vivo models of radiation-induced injury have been described, including cell culture models of irradiated endothelial cells18,19, fibroblasts20, or keratinocytes19 and in vivo rodent, swine, and non-human primate animal models. Rodent models are widely used in radiation research due to their similarities in response to radiation injury with humans and their flexibility of genetic manipulation21. Radiation dose requirements are higher in rodents than in humans when seeking similar outcomes: desquamation, fibrosis, and necrosis16,22. Description of scoring criteria to measure the response to radiation has further enhanced the adoption of rodent models of radiation skin injury21,23.
Current research in the preclinical setting focuses on understanding the mechanisms of radiation-induced skin injury and developing therapeutical options. Thus, establishing a robust and reproducible preclinical model to create the radiation insult with high clinical translatability is essential. This work describes a murine model of skin fibrosis with optimized radiation dose and delivery technique. Our model, which combines functional, histological, and molecular measurements, can be used to effectively study the mechanism of fibrosis development and investigate new therapeutical options.
Protocol
Ethical animal use was approved by the Institutional Animal Care and Use Committee (IACUC), which acts in compliance with the Animal Welfare Act. Animals were housed in an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) approved facility and treated according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
1. Anesthesia
- Place mice in the box of a small animal anesthesia system (Figure 1). Deliver 4% isoflurane to the box and wait for 5-10 min at which time lower the isoflurane to 2-3%.
- Confirm the anesthesia depth via toe pinch. Apply eye lubricant to prevent drying. Move the subject to the nose cone with 2% isoflurane flow.
- Use the above technique to anesthetize each mouse for shaving and limb measurement assay. Use an intraperitoneal pentobarbital injection dose of 1.25 mg/kg to anesthetize mice for irradiation.
2. Skin area preparation
- Plan to shave the mice 2-3 days before irradiating. Trim the hair using a clipper (Figure 2A).
- Apply depilatory cream and wait for 1-2 min (Figure 2B). Wipe the cream with dry gauze and rinse the skin with a phosphate buffer saline (PBS)-soaked gauze (Figure 2C).
3. Irradiation procedure
- Anesthetize each mouse with an intraperitoneal injection administered 5 min before irradiation. Position the limb in the radiation field (25 cm x 25 cm) and secure it with surgical tape (Figure 3A-C).
- Restrict the body using surgical tape (Figure 3D). Place a 1 cm thick bolus to prevent/minimize deep penetration of radiations (Figure 3E).
- Calculate the applicator and cutout factors to deliver 40 Gy to the mouse skin. For the experiment here, use a linear accelerator to generate a 6 MeV electron beam for 3740 monitor units to induce irradiation burns. In this setting, a 25 cm x 25 cm radiation field at 1,000 MU/min dose with 100 cm source to surface distance (SSD), which is the distance from the irradiation source to the top of the bolus to deliver the 40 Gy. Irradiation time will vary according to linear accelerator used, beam energy and field size. A radiation or medical physicist should be consulted to calculate the monitor units necessary to deliver 40Gy on the linear accelerator.
4. Visual monitoring of fibrosis development
- Use a handheld digital camera to document the progression of fibrosis. Use the macro setting to capture detailed photos.
- Anesethetize mice by inhalation of isoflurane.
- Take pictures by positioning the lens closer to the skin and press capture. Try to keep the photos as consistent as possible. Keep the subject under direct supervision until sufficient consciousness to maintain sternal recumbency is regained.
5. Measurement of leg excursion as a functional outcome of fibrosis
- Starting from third week after irradiation, plan to have measurements each week for up to 6 weeks.
- Initiate and maintain the anesthesia as described above. Prepare a field in front of the nose cone and fix a protractor with tape in the center (Figure 4A).
- Transfer the mouse to the field and gently place its nose on the cone. Position the right knee to the protractor's center (Figure 4B).
- Keep the knee fixed using the left hand and use the right hand to dorsiflex the foot with the index and pollex fingers (Figure 4C,D).
- Note the degree of extension by reading the value indicated by the toes. Perform the same procedure on the contralateral non-irradiated leg.
6. Measurement of gait functional index
- 3D-print the rodent walking track to create a 40 cm walking path suspended at a height of 15 cm with a transparent floor (Figure 5). Place a video recorder underneath the track and start filming. The camera is adjusted to record a video at the highest possible resolution and at a minimum frame rate of 60 frames per second.
- Open one end of the track and transfer the mouse inside. Let the animal walk freely on the track. Capture the animal walking as smoothly as possible at least three times.
- Check the quality of the video before recording the next mouse.
- Transfer the videos to a computer with a video player, screenshot application, an image processing program, and spreadsheet software. Watch the recordings to capture three different, clear footprints using the screenshot function (Figure 6).
- Open the image processing program for toe spread measurements. Select File from the upper panel and click Open to find and display the image to analyze.
- Select Straight Line Tool from the second row of the upper panel (Figure 7_1). Using this tool, mark the wall width and click Analyze > Set Scale, then enter the exact value for a known distance to calibrate the scale (Figure 7_2-5).
- Use the Straight Line Tool to mark the footprint for different measurements (Figure 6C, D; 1: foot length, 2: outer toe spread, 3: inner toe spread), select Analyze > Measure, and record the length value (Figure 8).
- Perform the analyses for both the irradiated and non-irradiated limbs. Use the following equation published previously to assess the functionality24

where SFI = sciatic functionality index, E = experimental or injured paw, N = normal or uninjured paw, TS = toe spread, and PL = print length.
7. Euthanasia
- Transfer the subject to a CO2 line-connected box. Start the CO2 infusion to reach 30%-70% concentration within the chamber. For a 5 L chamber, the gas infusion must be between 1.5-3.5 L/min.
- Wait for the animal to cease respiration for 5-10 min. Euthanize by cervical dislocation via holding the head in place from the skull base and firmly pulling the tail25.
8. Histology and downstream analyses 17
- Pull the irradiated hind limb to prepare it for excision. Select a 2 cm x 1 cm area to the posterior plane of the extremital skin.
- Use pair of sharp scissors to carefully collect the skin sample. Cut the tissue in half from the long axis to obtain two separate pieces.
- Fix one tissue piece in 10% formalin buffered saline. Process the fixed tissue for preparing histological sections on slides.
- Transfer the second piece to a box filled with dry ice to preserve the proteins and RNA. Then, quickly transfer the tissue samples to a -80 °C freezer and keep them frozen until further processing.
- Stain the slides for hematoxylin and eosin (H&E) stain and Masson's Trichrome stain. Visualize the stained slides under a microscope and take images using 10x magnification. Measure the epithelium thickness using image processing software as explained above for the gait functional assay.
9. Statistics
- Present the data as mean ± standard deviation. Evaluate the results using analysis of variance (ANOVA) followed by Bonferroni's multi-comparison test or Student t-test.
Results
Establishment and optimization of the current irradiation protocol resulted in a consistent and reproducible induction of fibrosis in mice. Right limbs of the mice were positioned and secured within the radiation field on the day of irradiation, and 40 Gy of radiation was administered.
The development of functional impairment in skin was monitored by capturing images every week, post-irradiation. Photos showed that the optimized protocol created fibrosis by day 40 with 95% confidence. An example of fibrosis progression, elucidated by a visible constriction of the irradiated skin, is shown in Figure 9.
Functional impairment is one of the most prominent features of radiation-induced fibrosis; as the fibrosis progresses, excessive remodeling of the extracellular matrix results in skin constriction and thickening. In addition, the radiation-induced reaction affects the function of the adjacent muscles. Authors established knee extension and video-based toe spread assays to quantify limb function. Application of a 40 Gy irradiation dose resulted in a significant decrease in the excursion of the irradiated leg compared to the contralateral non-irradiated leg by day 21 post-irradiation (Figure 10). This early result was predominantly related to acute reactions such as edema and dermatitis. Over time, earlier reactions subsided, and the range of motion improved but remained significantly lower than the baseline due to chronic reactions (fibrosis). This analysis was usually performed for up to 42 days post-irradiation (Figure 10).
Improving tissue function is one of the primary goals of therapeutic agents against radiation-induced fibrosis. The authors established a video-based toe spread assay to further measure the functional recovery. Irradiation of limbs resulted in a significant decrease in paw length and toe spread (Figure 11A,B). In addition, the functional index of the irradiated limb indicated a decrease in limb function (Figure 11C).
These functional assays are non-invasive and reflect a loss of tissue function post-irradiation attributed to early and late effects of the radiation damage. To draw a conclusive correlation between the functional assay outcome and fibrosis, we performed histological analyses of skin tissue 42 days post-irradiation. Hematoxylin and eosin (H&E) staining of the irradiated skin sections revealed a severely hyperplastic epidermis having deep rete pegs and the presence of parakeratotic crusts on the epithelial surface (Figure 12A, upper panel). Masson's trichrome staining showed excessive extracellular matrix deposition, a thickened epithelium, and denser connective tissue compared to non-irradiated skin sections (Figure 12A, lower panel). Quantitative measurement revealed a significant increase in epithelium thickness upon irradiation (Figure 12B). In conclusion, the functional tests combined with the histological analysis confirm the successful induction and functional evaluation of radiation-induced fibrosis in mice.

Figure 1: Small animal anesthesia system. Image of the small animal anesthesia system that can be used to anesthetize mice for different procedures. Please click here to view a larger version of this figure.

Figure 2: Pre-irradiation preparation. (A) Hair trimmed mouse: A representative mouse after hair trimming. (B) Mouse hair removal: Application of depilatory cream to remove hairs. (C) Hair-free limb: A representative hair-free mouse preparation before irradiation. Please click here to view a larger version of this figure.
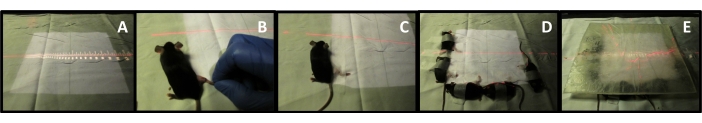
Figure 3: Hind limb irradiation setup. (A) Radiation field. (B) Limb abduction and body positioning. (C) Limb fixation. (D) Body fixation. (E) Bolus placement. Please click here to view a larger version of this figure.
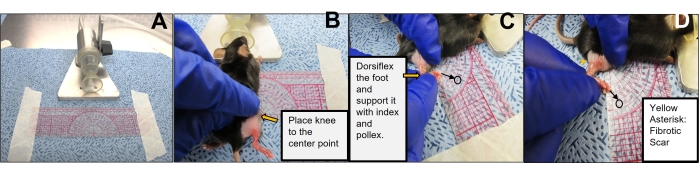
Figure 4: Limb excursion measurement. (A) Set up for measuring limb excursion. Image showing the arrangement of the protractor and anesthesia nose cone for performing the limb excursion measurement. (B) Positioning of the mouse for limb excursion measurement. The image is representative of how and where to place the mouse knee relative to the protractor for executing the limb excursion measurements. (C,D) Performance of mouse limb excursion. Images indicate the positioning of the hand to hold the limb and flexing directions to measure the degree of limb excursion. Please click here to view a larger version of this figure.
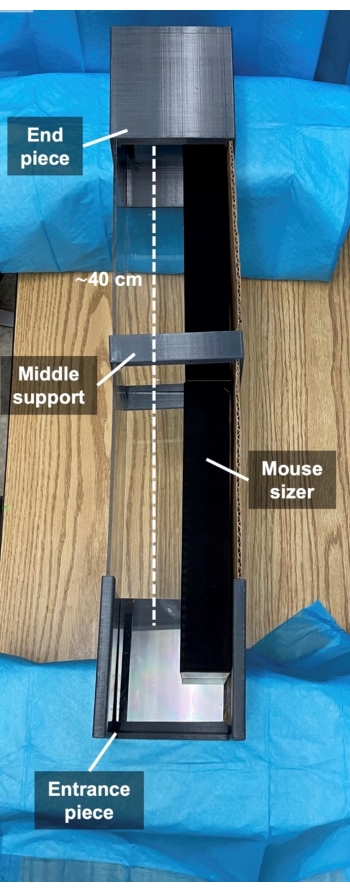
Figure 5: 3D printed mouse walking track. Representative image of 3D printed mouse walking track. Please click here to view a larger version of this figure.

Figure 6: Screenshots of mouse walking pattern. The subject was allowed to walk on the track and a video was captured from beneath. (A) Healthy mice footprint. (B) Irradiated mice footprint. (C) Healthy print measurements. 1: Foot length. 2: Outer toe spread. 3: Inner toe spread. (D) Irradiated print measurements. 1: Foot length. 2: Outer toe spread. 3: Inner toe spread. Please click here to view a larger version of this figure.
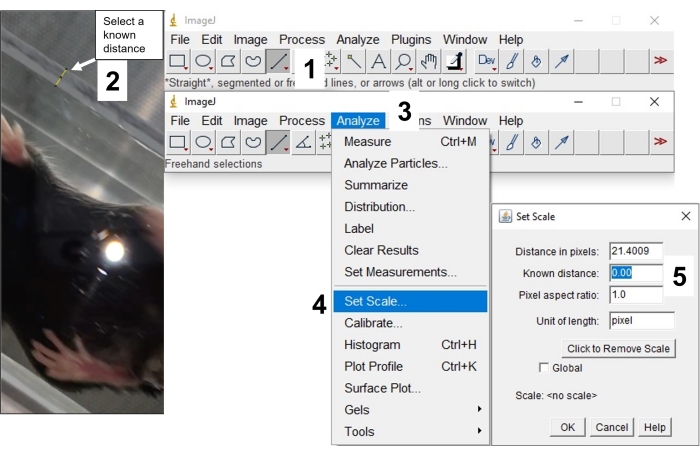
Figure 7: Scale calibration with image processing software. This image represents the steps to calibrate the scale. 1: Select straight selection tool, 2: Select a known distance in the photo, 3: Select analyze, 4: Click set scale, 5: Enter known distance. Please click here to view a larger version of this figure.
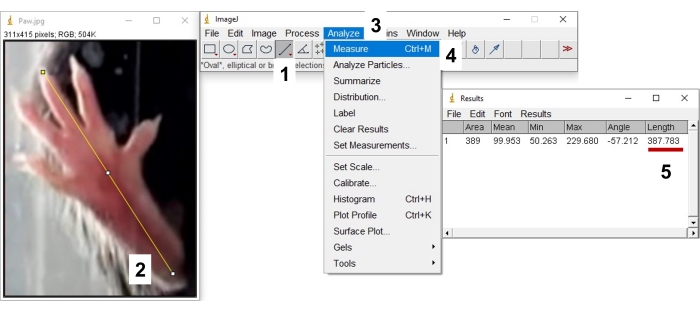
Figure 8: Measuring distances with image processing software. 1: Select straight selection tool, 2: Select distance, 3: Select analyze, 4: Select measure, 5: Record the distance. Please click here to view a larger version of this figure.
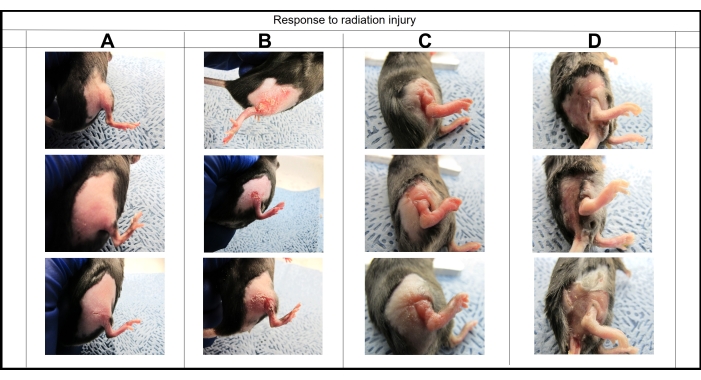
Figure 9: Visual monitoring of fibrosis progression. Images were taken at different time points to monitor the progression from erythema (A) day 3, dermatitis (B) day 9, edema (C) day 14, and fibrosis (D) day 42 following 40 Gy irradiation. Please click here to view a larger version of this figure.

Figure 10: Degree of motion. Representative graph of the change in the degree of limb motion at different time points post-irradiation (n = 6). ** p < 0.01, *** p < 0.001, **** p < 0.0001, and ns = not significant as compared with non-irradiated (NIR) control legs. The data present mean ± standard deviation. The results were evaluated using analysis of variance (ANOVA) followed by Bonferroni's multi-comparison test. Please click here to view a larger version of this figure.

Figure 11: Mouse walking track analyses. (A) Paw length: Graph representing the change in the paw length at day 42 post-irradiation (n = 14). * p < 0.05 as compared with non-irradiated control legs. (B) Toe spread: Graph representing the change in the toe spread at day 42 post-irradiation (n = 14). **** p < 0.0001 as compared with non-irradiated control legs. (C) Gait Index: Graph representing calculated gait function index at day 42 post-irradiation (n = 14). The data present mean ± standard deviation.The results were evaluated using an unpaired Student t-test. Please click here to view a larger version of this figure.
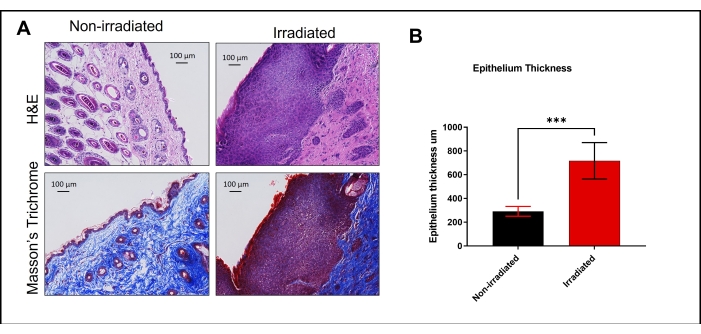
Figure 12: H&E and Masson's trichrome staining. (A) Histological sections of non-irradiated healthy skin and irradiated skin 42 days post-irradiation were stained with hematoxylin and eosin (H&E; upper panel) and Masson's trichrome stain (lower panel). Images were taken using 10x magnification. (B) Epithelium thickness was measured using image processing software (n = 5). *** p < 0.001 as compared with non-irradiated control epithelium thickness. The results were evaluated using an unpaired Student t-test. Please click here to view a larger version of this figure.
Discussion
Skin injury is a likely outcome of accidental or medical treatment-related exposure to radiation. Nuclear reactors possess an accidental breach risk due to human error or natural disasters like Chernobyl and Fukushima26,27. Therapeutical dosing for cancer treatment is the most common exposure, which uses fractionated repeated dose regimens that risk causing radiation-related fibrosis in the treated areas. This common chronic adverse reaction can be prevalent in up to 23% of the cases16,28. Therapeutic interventions directed against fibrosis should target the sequential pathological mechanisms involved during different stages of the disease. A cost-effective, reproducible, and translatable small animal model is ideal for further investigations. In this protocol, we described a murine model of radiation-induced injury that demonstrates pathological features of skin fibrosis.
Previous studies have shown similarities between the radiation-induced skin reactions and appearance between humans and mice16,21. The availability of a wide variety of strains and the genetically modified models that can help understand the molecular mechanisms of injury has made mice an ideal candidate for preclinical research. In addition, the radiation skin working group judged mouse models as the most rational first species for the screening studies16.
We administered a single dose of 40 Gy to irradiate the hind limb of mice to induce fibrosis. Irradiation resulted in visible erythema in earlier stages. In humans, erythema marks the earliest phase of the skin's reaction to irradiation15. Skin pigmentation and black hair color make the detection of erythema difficult; thus, hair removal before irradiation in the mouse model allows better documentation post-irradiation. Hind limbs can be abducted to have isolated radiation administration, which is crucial because pelvic or abdominal radiation can be fatal. Additionally, mice are less dependent on their hind limbs, which allows the researchers to work with the animals without causing excessive discomfort or fatality. Any vital organ irradiation will increase fatality, and tail irradiation can cause amputation and pain.
Radiation dose selection is essential to maintain the reproducibility of the described model. We tested a dose range of 30-45 Gy in our studies, and the results revealed that the pathological effects are inconsistent at lower doses, while higher doses had high mortality. Based on this experience, we decided on a single dose of 40 Gy to be optimal for achieving reproducibility and minimal lethality. Clinical radiotherapy regimens utilize a fractioned accumulation of radiation to prevent side effects; replicating this method in animal models could be more relevant. However, the use of fractionated doses in mice does not result in reproducible and clinically similar fibrosis-related pathological features. There are significant differences between mice and humans, yet they are highly relevant in radiation response in the skin16,21. The histological staining analysis confirmed that we can achieve characteristics of radiation fibrosis comparable in humans with this single-dose murine model. Authors observed an increase in epithelial thickness and dermal extracellular matrix deposition upon irradiation. In scenarios of accidental radiation events, exposure to doses ranging from 20-50 Gy from the fallout is possible. Due to the excess of the cellular papillae, mouse skin usually requires double the radiation dose to have a comparable clinical outcome to human skin. Combined thermal or mechanical trauma can further complicate the healing process and worsen fibrosis. Additional mouse models involving a combination injury must be investigated to mimic accidental and war scenarios. Bolus application focuses the radiation dose on the skin and reduces penetration to the bone and muscle. In the absence of bolus, we observed more severe effects in limb function and higher mice lethality rate. This observation correlates with the real-world clinical scenario; point-of-no-return extensive skin damage impairs the function of other organs and organ systems9,29.
Skin contracture and muscle and nerve injury due to late-stage fibrosis significantly reduce the quality of life of patients. The mouse model includes measuring limb excursion and gait function as a functional metric of fibrosis. Limb motion range is significantly reduced after irradiation. The use of a range of motion is a well-accepted endpoint outcome of the fibrotic contracture30. In humans, late-stage fibrosis can develop within a couple of months to several years. In this study, earlier effects of radiation caused excessive restriction, yet resolved with time. Contrarily, late effects such as fibrosis remained unresolved and were prominent in visual, functional, and histological assessments. General restriction persisted up to day 46, the furthest experimental endpoint.
Walking track analysis has been reliably used to determine the functional recovery from nerve injury-related muscular function. It is a direct metric of functional capacity24. The sciatic nerve defect results in defective plantar flexion represented as extended print length and reduced toe spread. The posterior tibial nerve defect representation is similar to the sciatic nerve defect, while the peroneal defect in mice is not well presented compared to rats24. Commonly used methods include ink-soaked footprints24, which can be difficult to control and operator unfriendly. We modified the original protocol to use a transparent walking track, recorded mice gait and walking patterns, took snapshots of paw prints, and measured the toe spread and palm length with an image processing software.
Toe spread and a gait index was added to corroborate the results obtained from the limb excursion assay. Authors plotted the values of the toe spread and palm length, or calculated the gait functional index, using the values described by Inserra et al. for the sciatic functional index24. We observed that irradiation results in a significant decrease in these indices correlating with the loss of limb excursion. In conclusion, walking track analysis can be confidently used to measure limb function in mice.
Irradiated skin can be excised post-sacrifice and processed for histological and molecular analyses. Histologically, the skin sections can be stained with H&E and Masson's Trichrome stain to assess inflammation and extracellular matrix deposition. In addition, collagen and elastin-based immune staining can be used to evaluate deposition. Immunohistological analyses can also be performed to analyze proteins of interest. Lastly, tissue can be analyzed for gene expression using real-time quantitative PCR or protein expression using either the ELISA technique or Multiplex Luminex assay.
The described method induces reliable superficial radiation injury on hind limbs without causing excessive fatality risk. Authors observed the markers of chronic fibrosis after radiation exposure as: excessive matrix deposition, epithelial thickening, and chronic expression of fibrotic markers17. Previous studies used different application techniques and radiation doses to induce radiation injury. The radiation dose and method of administration to induce fibrosis have not been validated before. However, based on results from this study and previously published work, the use of this method can be a highly valuable tool in radiation research.
One of the limitations of this model is that fractional dose application does not result in reproducible pathological features. The use of a protractor guided general range of motion measurement was not validated in radiation induced fibrosis. However, fibrotic expression affects knee motion. This functional assessment is valuable to track late effects of radiation injury. It is important to train personnel beforehand, and that all measurements are conducted by the same person. Gait index was primarily identified to assess the nerve function following nerve injury. The method was not validated to assess recovery following radiation-induced fibrosis. However, the overall fibrosis of the limb affects the gait. Benefits of the method to assess gait provides valuable information as an adjunct to the commonly used metrics to assess functional impairment in radiation-induced fibrosis, such as degree of motion. The described method of measuring the paw length from recorded videos is an additional read-out.
Supporting cumulative observations showed that the defined protocol could be reliably used to study radiation injuries and assess the effects of therapeutics.
Disclosures
The authors have no competing financial interests or other conflicts of interest related to this work.
Acknowledgements
This work is funded by research grants from the Department of Defense W81XWH-19-PRMRP-DA, NIAID/NIH Grant 5R21AI153971-02, and PSF/MTF Grant 603902.
Materials
| Name | Company | Catalog Number | Comments |
| 10% Formalin | Fischer Scientific | 23-427098 | |
| Bolus | Orfit | 8333.SO1/R | |
| Clipper | Kent Scientific Corp. | CL8787-KIT | |
| CO2 | Various | ||
| CO2 Chamber | E-Z Systems Inc. | E-22000 | |
| Depilatory Cream | Church & Dwight Co., Inc. | Nair | |
| Digital Camera | Wolfang | GA100 | |
| Eppendrof Tubes | Eppendorf | 22364111 | |
| Eye Lubricant | Dechra | Puralube Ophthalmic Ointment | |
| Gauze | Covidien | 682252 | |
| Image Processing Program | NIH | Image J | |
| Isoflurane | Dechra | USP Inhalation Anesthetic | |
| Linear Accelaerator | Varian Medical Systems, Inc. | 23EX | |
| PBS | Cytiva | SH30256.LS | |
| Pentobarbital | Akorn Pharmaceuticals | Nembutal | |
| Protractor | Westcott | 550-1120 | |
| Small Animal Anesthesia System | E-Z Systems Inc. | EZ-SA800 | Single animal system |
| Spreadsheet Software | Microsoft | Excel | |
| Surgical Scissors | Medline | MDS0834111 | |
| Surgical Tape | 3M | 1538-1 | |
| Tape | 3M | H-1113 |
References
- Breitkreutz, D., Mirancea, N., Nischt, R. Basement membranes in skin: unique matrix structures with diverse functions. Histochemistry and Cell Biology. 132 (1), 1-10 (2009).
- Kim, J. -. S., et al. Comparison of skin injury induced by β-and γ-irradiation in the minipig model. Journal of Radiation Protection and Research. 42 (4), 189-196 (2017).
- Kolarsick, P. A., Kolarsick, M. A., Goodwin, C. Anatomy and physiology of the skin. Journal of the Dermatology Nurses' Association. 3 (4), 203-213 (2011).
- von Essen, C. F. Radiation tolerance of the skin. Acta Radiologica: Therapy, Physics, Biology. 8 (4), 311-330 (1969).
- Dainiak, N., et al. Literature review and global consensus on management of acute radiation syndrome affecting nonhematopoietic organ systems. Disaster Medicine and Public Health Preparedness. 5 (3), 183-201 (2011).
- Hopewell, J. The skin: its structure and response to ionizing radiation. International Journal of Radiation Biology. 57 (4), 751-773 (1990).
- Flynn, D. F., Goans, R. E. Nuclear terrorism: triage and medical management of radiation and combined-injury casualties. Surgical Clinics. 86 (3), 601-636 (2006).
- Peter, R. Cutaneous radiation syndrome in multi-organ failure. The British Journal of Radiology. 78 (1), 180-184 (2005).
- Meineke, V. The role of damage to the cutaneous system in radiation-induced multi-organ failure. The British Journal of Radiology. 78 (1), 95-99 (2005).
- Berger, M., Christensen, D., Lowry, P., Jones, O., Wiley, A. Medical management of radiation injuries: current approaches. Occupational Medicine. 56 (3), 162-172 (2006).
- Ralf, U. P., Petra, G. Management of cutaneous radiation injuries: diagnostic and therapeutic principles of the cutaneous radiation syndrome. Military Medicine. 167, 110-112 (2002).
- Bray, F. N., Simmons, B. J., Wolfson, A. H., Nouri, K. Acute and chronic cutaneous reactions to ionizing radiation therapy. Dermatology and Therapy. 6 (2), 185-206 (2016).
- Mendelsohn, F. A., Divino, C. M., Reis, E. D., Kerstein, M. D. Wound care after radiation therapy. Advances in Skin & Wound. 15 (5), 216-224 (2002).
- Peter, R. U. . Radiation Treatment and Radiation Reactions in Dermatology. , 185-188 (2015).
- Ejaz, A., Greenberger, J. S., Rubin, P. J. Understanding the mechanism of radiation induced fibrosis and therapy options. Pharmacology & Therapeutics. 204, 107399 (2019).
- Williams, J. P., et al. Animal models for medical countermeasures to radiation exposure. Radiation research. 173 (4), 557-578 (2010).
- Ejaz, A., Epperly, M. W., Hou, W., Greenberger, J. S., Rubin, J. P. Adipose-derived stem cell therapy ameliorates ionizing irradiation fibrosis via hepatocyte growth factor-mediated transforming growth factor-beta downregulation and recruitment of bone marrow cells. Stem Cells. 37 (6), 791-802 (2019).
- Haubner, F., et al. Effects of external radiation in a co-culture model of endothelial cells and adipose-derived stem cells. Radiation Oncology. 8 (1), 66 (2013).
- Ebrahimian, T. G., et al. Cell therapy based on adipose tissue-derived stromal cells promotes physiological and pathological wound healing. Arteriosclerosis, Thrombosis, and Vascular Biology. 29 (4), 503-510 (2009).
- Haubner, F., et al. A co-culture model of fibroblasts and adipose tissue-derived stem cells reveals new insights into impaired wound healing after radiotherapy. International Journal of Molecular Sciences. 16 (11), 25947-25958 (2015).
- Urano, M., Kenton, L. A., Kahn, J. The effect of hyperthermia on the early and late appearing mouse foot reactions and on the radiation carcinogenesis: effect on the early and late appearing reactions. International Journal of Radiation Oncology Biology Physics. 15 (1), 159-166 (1988).
- Law, M., Thomlinson, R. The pathogenesis of necrosis after radiotherapy. The British Journal of Radiology. 47 (562), 740 (1974).
- Abe, Y., Urano, M. Fraction size-dependent acute skin reaction of mice after multiple twice-a-day doses. International Journal of Radiation Oncology Biology Physics. 18 (2), 359-364 (1990).
- Inserra, M. M., Bloch, D. A., Terris, D. J. Functional indices for sciatic, peroneal, and posterior tibial nerve lesions in the mouse. Microsurgery. 18 (2), 119-124 (1998).
- Suckow, C. P. S. a. M. A., Weichbrod, R. H., Thompson, G. A., Norton, J. N. . Management of Animal Care and Use Programs in Research, Education, and Testing. , (2018).
- Yamashita, S., Suzuki, S., Suzuki, S., Shimura, H., Saenko, V. Lessons from Fukushima: latest findings of thyroid cancer after the Fukushima nuclear power plant accident. Thyroid. 28 (1), 11-22 (2018).
- Cardis, E., et al. Cancer consequences of the Chernobyl accident: 20 years on. Journal of Radiological Protection. 26 (2), 127-140 (2006).
- Williams, N. R., et al. Radiation-induced fibrosis in breast cancer: A protocol for an observational cross-sectional pilot study for personalised risk estimation and objective assessment. International Journal of Surgery Protocols. 14, 9-13 (2019).
- Meineke, V., Fliedner, T. Radiation-induced multi-organ involvement and failure: challenges for radiation accident medical management and future research. The British Journal of Radiology. 78 (1), 196-200 (2005).
- Stone, H. B. Leg contracture in mice: an assay of normal tissue response. International Journal of Radiation Oncology Biology Physics. 10 (7), 1053-1061 (1984).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved