Method Article
Live Fluorescence, Inverse Imaging of Cell Ruffling, and Macropinocytosis
In This Article
Summary
This protocol demonstrates dextran imaging in live cells using continuous uptake and inverse images to optimize visualization of ruffling, macropinosome maturation, and analysis of dextran and other cell labelings.
Abstract
Macropinocytosis is a highly conserved but still incompletely understood process that is essential for the uptake and ingestion of fluid, fluid-phase nutrients and other material in cells. The dramatic extension of cell surface ruffles, their closure to form macropinosomes, and the maturation of internalized macropinosomes are key events in this pathway that can be difficult to capture using conventional confocal imaging based on tracking a bolus of fluorescent cargo. Fluorescent dextrans are commonly used experimentally as fluid phase markers for macropinosomes and for other endocytic pathways. A method the lab has adopted to optimize the imaging of dextran uptake involves using live imaging of cells bathed in high concentrations of fluorescent dextran in the medium, with the unlabeled cells appearing in relief (as black). The cell ruffles are highlighted to visualize ruffle closure, and internalized macropinosomes appear as fluorescent vacuoles in the cell interior. This method is optimal for visualizing macropinosome features and allows for easy segmentation and quantification. This paper describes dual-labeling of pathways with different sized dextrans and the co-expression of lipid probes and fluorescent membrane proteins to demark macropinosomes and other endosomes. The detection of internalized dextran at an ultrastructural level using correlative light and electron microscopy (CLEM) is also demonstrated. These cell processes can be imaged using multiple live imaging modalities, including in 3D. Taken together, these approaches optimize macropinosome imaging for many different settings and experimental systems.
Introduction
Most vertebrate cell types share the innate capacity for the non-selective uptake of fluid with predecessors throughout evolution, dating back to single-cell amoeba1. This highly conserved process of fluid-phase uptake by macropinocytosis (big drinking) is used by amoeba primarily for nutrient acquisition1, while in vertebrate cells, it can similarly be a supply route for obtaining nutrients under stress, and it is used by immune cells for the sampling and surveillance of tissue environments2. Macropinocytosis has features that distinguish it from other forms of endocytosis, including the distinctive, large (>0.2 mm diameter) vacuolar-like macropinosomes, the non-selective nature of fluid and solute uptake and the accompanying expanses of plasma membrane that are opportunistically internalized into macropinosomes3. The non-selective nature of this uptake means that sorting and recycling must occur rapidly to rescue essential soluble and plasma membrane proteins by recycling them back to the cell surface. Much of the material taken into macropinosomes is destined for degradation in lysosomes, and it is funneled into endo/lysosomal pathways through macropinosome maturation and fusion with other endosomes. Plasma membrane proteins can also be recycled back to the cell surface from macropinosomes. There is intense interest in the cancer field for understanding the process of macropinocytosis, which is activated in cancer cells and helps to sustain their nutrition and proliferation2,4,5,6. Macropinocytosis occurs both constitutively and can be further induced upon receptor-mediated stimulation in immune cells, where macropinosomes host receptor signaling, contribute to endo/lysosomal processing for antigen presentation and provide an entry portal for a variety of viruses, bacteria, and other pathogens3,7.
Macropinosomes have few distinctive or unique markers. They are most easily identified during their formation at the base of dramatic, actin-rich cell surface ruffles, which close to engulf fluid8. Immediately after closure, the F-actin around macropinosomes is depolymerized, and the macropinosomes themselves undergo dynamic changes associated with homo- and heterotypic fusion, tubulation, and shrinkage9. Macropinosomes are also difficult to identify at an ultrastructural level since they have no characteristic coats or other features and appear simply as vacuoles of varying sizes. Without definitive membrane markers, macropinosomes are most readily and traditionally defined by their fluid cargo, which experimentally is through the use of fluorescent dextran. Dextran is a glucose-derived complex polysaccharide that can be coupled to a large array of fluorescent or electron-dense markers and added to the medium for uptake by cells or perfused for uptake in tissues. Moreover, large molecular weight (MW) dextran (70 kDa) is now regarded as a size-selective cargo for macropinosomes since it does not enter other, smaller endocytic vesicles, and it has become the most common label for macropinocytosis10,11. Viewing macropinocytosis typically involves incubating cells with a pulse of fluorescent dextran and using fixed or live cells for fluorescence imaging to view the bolus of dextran labeling inside cells in macropinosomes. Due to their large size, unlabeled macropinosomes can also be viewed in some cell types using phase contrast or bright-field microscopy, wherein macropinosomes appear as large white empty vacuoles. Some contextual information is gained from the phase-contrast images of the cells, and these images can then additionally be overlayed with fluorescence images to depict fluorescent dextran uptake12,13. Dextrans of different sizes and with distinct labels can be used to monitor fluid uptake into multiple cellular and endocytic pathways10,14,15 and dextran can be applied to cell cultures or perfused into mice for intra-vital imaging16 or even injected into fly embryos17 for live imaging.
Some of the difficulties incurred in using dextran to track macropinosomes include its leakage out of macropinosomes after fixation or permeabilization of cells, it's dilute or hard-to-detect levels in cells that do not perform aggressive macropinocytosis, and its dynamic deployment and dispersion inside cells during macropinosome maturation3. These issues are compounded in most experiments which are designed to track the uptake of a single pulse of dextran added to cells and then washed off.
Instead, this paper describes the advantages of applying fluorescent dextran to the cells and leaving it in the medium during live imaging to record continuous uptake into cells. This amplifies the dextran signal in macropinosomes and subsequent endosomes showing the whole pathway of maturation. Viewing labeled macropinosomes against the unlabeled (black) interior of the cells, as a high contrast inverse setting, reveals all the features of the macropinosomes and, conversely, outside the cells, the highly fluorescent medium highlights the dynamic ruffling of the cell surface. This method describes the live imaging of dextran for light microscopic, confocal imaging, and for its detection after cell fixation by correlative light and electron microscopy (CLEM). The imaging included here was performed on different cell types to demonstrate the broad applicability of these approaches, including activated macrophages and microglial cells, where macropinocytosis is very active and rapid, and in cancer cells where macropinocytosis is relatively less abundant.
Protocol
1. Preparation of cells on 35 mm glass-bottom dishes (Day 0)
- Maintain and passage cell lines in complete medium supplemented with 10% heat-inactivated (for RAW264.7) or regular fetal calf serum and 1% L-glutamine, at 37 °C in humidified 5% CO2 incubator.
- Plate the appropriate number of cells to achieve 60% confluency in 24 h, on 35 mm glass-bottom dishes.
NOTE: The recommended cell density is between 2 mL of 0.15 x 106 cells/mL to 0.25 x 106 cells/mL for the RAW264.7, BV2 microglial cells, and MDA-MB 231 breast cancer cells described here.
2. Fluorescence DNA plasmid transfection (Day 1) - Optional
NOTE: Different fluorescently-tagged proteins and probes can be expressed transiently or stably in cloned cell lines to specifically label actin ruffles and compartments in the endocytic pathway such as early endosomes, late endosomes, or lysosomes.
- Transfect the cells using a lipid-based transfection kit according to the manufacturer's instruction, 1 day post plating in a tissue culture hood.
- Dilute 2 µg of endotoxin-free purified DNA plasmid in 125 µL of minimum essential media (reduced serum). Mix gently and let it stand for 5 min at room temperature.
- Dilute 5 µL of transfection agent in 125 µL of minimum essential media (reduced serum). Mix gently and let it stand for 5 min at room temperature.
- Combine the diluted DNA plasmid and the diluted transfection reagent and incubate for another 10 min before adding dropwise to cells.
- Incubate the cells with transfection complex at 37 °C in a humidified 5% CO2 incubator for 3-4 h, before replacing with fresh full culture medium.
- Use the transfected cells the next day for experiments or subject to limited dilution cloning to obtain stably expressing cell lines.
3. Visualization of endocytic vesicles and macropinosomes (Day 2)
NOTE: Fluorescent dextrans (Dextran Alexa Fluor 488/647 at 10 kDa MW, and Dextran Oregon Green 488/Tetramethylrhodamine at 70 kDa MW, Anionic, Lysine fixable) of different molecular weights are used to monitor fluid-phase uptake into a range of endocytic pathways14. 70 kDa dextran for macropinosomes and 10 kDa for all pathways.
- Prepare dextran(s) as a 2x concentrated suspension at 200-500 ng/mL in pre-warmed culture medium for addition to cells.
- OPTIONAL: Experiments may require prior or simultaneous addition of supplements or drugs to influence endocytic activity. For instance, pre-treat macrophages and microglial cells with growth factors (e.g., 200 ng/mL CSF-1) or activating ligands (e.g., LPS 100 ng/mL or 300 ng/mL CpG) to enhance ruffling and macropinocytosis. Culture the cancer cells in an appropriate serum-free medium for 12-16 h before dextran uptake in the complete medium. Refer to step 1.2 for recommended cell density.
- To prepare for live imaging, position the cells grown in glass-bottom dishes on the stage of an inverted confocal microscope fitted with 37 °C heating pad and a 5% CO2 humidified incubator.
- Aspirate the excess medium leaving 500 µL of the complete medium in the glass bottom dish.
- Find a suitable region of cells and set focus. For time-lapse imaging, pre-select the appropriate fluorescence lasers, filters, and settings before acquisition. Use the following settings to resolve individual cells and macropinosomes.
- Image the region of interest on an inverted confocal microscope with a 63x 1.4 NA oil immersion Plan Apochromat objective.
- Select 512 x 512 for image size, bidirectional scanning for fast imaging.
- Focus on an individual focal plane of the cells. Set to capture as a single slice or set an optical slice thickness (Nyquist) of approximately 0.3 µm to combine as Z-stacks.
- Select time-lapse with 5 s interval, for a duration between 20-45 min depending on specific cell types.
- Select hardware auto-focusing/focus tracking to aid in image stabilization during capture for an appropriate duration according to the specific cell types.
- Add an equal volume of 2x dextran spiked medium and start live image capture immediately for macrophages.Wait 20 min before imaging the slower uptake in MDA-MB 231 cells.
NOTE: With this method, the BV2 cells with two fluorophore channels were successfully imaged on a single plane with maximum speed tracking, continuously for 45 min upon addition of the dextran mixture. To detect early macropinosomes in RAW 264.7 cells, where macropinocytosis occurs very rapidly, acquire a single slice (for fast-imaging) or Z stack of 4-6 slices (for 3D capture) and time-lapse with 5 s interval for 20 min upon the addition of dextran. For MDA-MB 231 cells, acquire optimal Z-stacks between 10-15 slices, with 5 s intervals for 40 mins.
4. Correlative light and electron microscopy (CLEM) (Day 3) - Optional
- Prepare cells according to steps 1, 2, and 3 of the protocol with adaptations. In brief, grow 0.2 x 106 cells/mL RAW264.7 cells on gridded glass-bottom dishes (35 mm Dish, No. 1.5 Gridded coverslip, 14 mm glass diameter, uncoated), transfect with a fluorescent plasmid (e.g., mCherry-2xFYVE) and leave overnight before the addition of dextran-488 (70 kDa) for 20 mins at 37 °C, 5% CO2 humidified incubator.
- Wash the cells swiftly with 1 mL of ice-cold PBS and fix with 0.1% glutaraldehyde.
- Acquire the fixed cell images of the dextran-488 and mCherry-2xFYVE on a confocal microscope.
- Capture bright-field images to identify the grid positions before the dishes are processed for transmission electron microscopy (EM) as previously described18. In brief, further, fix cells in 2.5% glutaraldehyde, postfix in 1% reduced osmium tetroxide, en-bloc stain with 2% uranyl acetate, and then dehydrate through a series of ethanol before embedding in LX112 resin. Excise the gridded locations from the block and collect ultra-thin sections using an ultra-microtome. Capture images on an electron microscope at 80 kV using appropriate imaging system software.
5. Data processing and visualization (Day 4)
NOTE: FIJI-an open-source image analysis package-is used for visualization and analysis33.
- Duplicate the raw data for processing to comply with F.A.I.R. data principles, i.e., that research data is Findable, Accessible, Interoperable, and Reusable.
- Adjust the brightness and contrast for image sets equally using the histogram data.
- Display the images as maximum intensity projections from Z-stack images acquired.
- Insert scale bar for image sets.
- Crop/Rotate FOV data (optional). Create representative panels for the time series data.
- For further analysis and quantification, quantify the fluorescence intensity of dextran taken up into cells as a measure of macropinocytosis activity. To confine the analysis to individual cells, use the imaging method described here.
- Invert the LUT to flip the color for cell areas devoid of fluorescence to perform thresholding.
- Segment the foreground object and create a cell mask to identify individual cells.
- Use the cell mask in conjunction with a method19 previously published that identifies and measures individual macropinosomes. Use these metrics to quantify macropinocytosis, including size, number, and total uptake per cell.
Results
The approach of imaging unlabeled live cells immersed in a high concentration of fluorescent dextran has several advantages over conventional imaging techniques for tracking macropinocytosis. By presenting the images in relief, the cell bodies appear as black and allow improved visualization of the dramatic cell surface ruffling against a bright, fluorescent background, followed by fluid-phase internalization of TMR dextran (70 kDa)-filled macropinosomes as depicted in Figure 1A. In this maximum intensity projection, a cancer cell is recorded with macropinosomes entering the cells from the ruffling borders. The black interior of the cell, with no background or out-of-focus fluorescence, provides for high contrast definition of the macropinosomes, precisely depicting their size and shape and revealing changes that occur during maturation as depicted in Figure 1B and highlighted in movie sequences in Figure 1C. Homotypic fusion accompanies increases in size (Figure 1C), and tubulation of macropinosomes can be clearly seen (Figure 1D). Hence, we are now able to image the trajectory of fluid cargo moving into the cell in macropinosomes and through the successive maturation steps. Live imaging abolishes the need for any chemical fixation steps that can cause structural alterations to these vesicles. An additional advantage is that laser intensities can be reduced, and the relief of the cellular outlines negates the need for an additional whole cell marker.
In Figure 2, cells are bathed in a mixture of two fluorescent dextrans, 647-dextran (10 kDa) and 488-dextran (70 kDa), as fluid phase markers. The brightly fluorescent medium contains both dextrans; hence, in the merged image, the medium is yellow, and the cells contain mixtures of red, green, and yellow labeled endosomes. The red channel shows uptake into large macropinosomes and into a range of smaller endosomes, some of which have very bright 647-dextran (10 kDa), indicating the concentration of the cargo in these endosomes. In the green channel, only larger macropinosomes are labeled and distinguished by having 488-dextran (70 kDa) as luminal cargo, which notably is at the same intensity as the extracellular medium, indicative of the non-selective gulping typical of macropinocytosis. This approach provides both the means to identify macropinosomes but simultaneously displays other endosomes for comparison and the potential for quantifying these pathways and their points of intersection.
In Figure 3, transfected cells are used to express fluorescent cell markers for contextual information in cells undergoing uptake of 488-dextran (70 kDa) from the medium. Fluorescently tagged probes are used here to depict membrane phospholipids during macropinocytosis. mCherry-PLCδ-PH is a fusion protein of the pleckstrin homology domain from PLCδ and the mCherry fluorescent protein20, which is a specific probe for phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) in the plasma membrane (Figure 3A). mCherry-2xFYVE is an FYVE domain-containing probe specific for phosphatidylinositol 3-phosphate (PI3P) on early endosomes21 (Figure 3B).
In Figure 3A, mCherry-PLCδ-PH highlights an enrichment of (PI(4,5)P2) on plasma membrane ruffles up to the point of macropinosome closure. However, it is rapidly removed from macropinosomes moving into the cell. The separation of dextran-filled macropinosomes as they internalize from the labeled plasma membranes is clear in this example.
As dextran-filled macropinosomes mature further and fuse with early endosomes, they acquire PI3P, which can be labeled in a circumferential fashion with mCherry-2xFYVE, as shown by correlative light and electron microscopy (CLEM), which provides the opportunity to further interrogate labeled compartments at an ultrastructural level (Figure 3B). The CLEM image shows two-color fluorescence superimposed on the ultrastructural view of the cells, allowing contextual analysis of the endosomes and cell interior. In the image depicted here, the mCherry-2xFYVE can be seen surrounding dextran-filled macropinosomes (M), which by EM appear as large empty vacuoles. This dextran has not yet reached late endosomes/lysosomes (L), which are unlabeled at the fluorescence level but can be seen by EM and identified by their intraluminal vesicles.
Another example of combined imaging of fluorescently-labeled cargos is shown in BV2 microglial cells, pre-starved in serum-free medium and then treated simultaneously with 647-Transferrin and 488-dextran (70 kDa) (Figure 4). 647-Transferrin binds to surface transferrin receptors which are internalized and recycled back to the surface from punctate22. The (red) recycling endosomes contrast with the dextran-filled macropinosomes (green), and this can even be found clustering together for the possible intersection of pathways. This figure demonstrates live fluorescence images overlayed on bright-field images to view cell features. Macropinosomes are shown as inverse images in the Figure 4 insets. Frames over an ~7 min time course track the internalization of dextran-filled macropinosomes and their movement from the ruffles toward the perinuclear region.

Figure 1: Use of single fluorophore-conjugated dextran and negative relief live cell imaging to visualize macropinocytosis. (A) MDA-MB 231 cells were incubated in serum-free media for 16 h and then pre-treated for 20 min with TMR- dextran 70 kDa MW in full media, and a 40 min time-lapse video was then taken to label and visualize macropinosome trafficking over time. Representative max-intensity projections of live cell microscopy at a single time point are provided in Red and Lut-Gray. The yellow and pink box areas are enlarged in C and D to show successive events of macropinosome fusion and tubulation. (B) Schematic illustration of imaging unlabeled live cells in relief, immersed in fluorescent dextran to track the trajectory of fluid cargo moving into the cell and through the macropinocytic/endo/lysosomal pathway. (C) Yellow insert boxes and arrows highlight the uptake of dextran cargo into small macropinosomes that can fuse and grow in size within the cell. t' = 0 denotes the start of this segment focused on fusion, extracted from a longer movie. (D) Pink insert boxes and arrows highlight tubulation of macropinosomes. t" = 0 denotes the start of this segment focused on tubulation selected from a longer movie. Scale bar: 10 µm. Please click here to view a larger version of this figure.
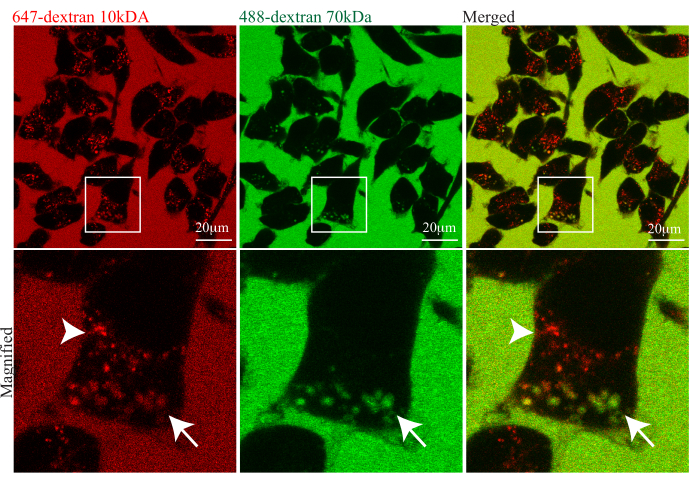
Figure 2: Use of dual fluorophore-conjugated dextrans and negative relief live-cell imaging technique to distinguish trafficking in different endocytic pathways. BV2 microglial cells were immersed in a high concentration of two dextrans (647-dextran 10 kDA and 488-dextran 70 kDa MW) and imaged continuously over time. Representative live-cell images reveal macropinosomes that are labeled green or yellow when they contain both 10 kDa and 70 kDa dextran (as indicated by the white arrow), and other smaller endosomes are labeled only with 10 kDa dextran (red) (as indicated by the white arrowhead). The white boxes are enlarged in the lower panel to highlight the different populations of endocytic vesicles. Scale bar: 20 µm. Please click here to view a larger version of this figure.

Figure 3: Use of additional fluorescence labels and ultrastructural analysis. (A) RAW 264.7 cells were transiently transfected with mCherry-PLCδ-PH to demarcate PI(4,5)P2 on the plasma membrane. Representative live-cell images demonstrated that under LPS stimulation, PI(4,5)P2 is enriched on the plasma membrane and recruited initially to ruffles. Macropinosomes (labeled green by 488-70 kDa dextran; white arrow) separate from the plasma membrane as they internalize and lose PI(4,5)P2. t' demonstrates time point from ruffling events to the uptake of dextran into the cell. Scale bar: 10 µm. (B) Macrophages were grown on gridded glass-bottom dishes and transfected with mCherry-2xFYVE to label PI3P in mature macropinosomes/early endosomes. 488-70 kDa dextran was added to cells for 20 min before fixation with 0.1% glutaraldehyde, imaged with confocal microscopy, and then cells were processed for transmission electron microscopy using standard techniques. EM images were captured using appropriate imaging system software. CLEM images were combined and aligned with confocal images manually to depict fluorescence labels and ultrastructural features simultaneously. M: macropinosome. L: late endosome/lysosome. Scale bar: 2µm. Please click here to view a larger version of this figure.

Figure 4: Imaging live uptake of dextrans alongside other fluorescent cargo. BV2 microglial cells were pre-starved with serum-free medium for 1 h before treatment with a molecular probe, 647-Transferrin as a marker to label cell surface transferrin receptor and track its course through recycling endosomes for comparison with macropinosomes labeled green by 488-70 kDa dextran. t" represents the time point where images were taken from the time-lapse.Representative live-cell images demonstrated internalized 647-Transferrin clusters in punctate recycling endosomes (as indicated by the white arrows), some of which are associated with green macropinosomes. The white boxes are enlarged in the right panels to depict details of transferrin clusters at macropinosomes. A superimposed bright-field channel allows visualization of membrane ruffles. Scale bar: 10µm. Please click here to view a larger version of this figure.
Discussion
This paper describes variations on more traditional ways of using dextran labeling to track macropinocytosis, based on live imaging of cells bathed continuously in fluorescent dextran(s) and visualizing the uptake on the black background of unlabeled cells. The optimized protocol provides the means to distinguish between different intracellular vesicles and allows a spatial-temporal tracking of multiple macropinocytotic and endocytic cargos and proteins. The method of immersing cells in a dextran medium to monitor the dynamic process of fluid-phase uptake was first demonstrated in Dictyostelium23. Examples of other previous studies have also viewed cells in relief to view uptake15,16. The protocol described in this paper provides a more comprehensive treatment and analysis of this approach that will now facilitate future studies in a wide variety of cell and tissue settings. This approach is also amenable to organoids bathed in dextran and to in vivo settings where dextran is usually perfused or injected into tissues, embryos, and whole organisms where it cannot easily be washed out.
The uptake of small and large dextrans, separately or together, can provide a powerful setting for quantification and for comparing macropinocytosis with other endocytic pathways. In dual labeled samples, populations of endosomes can be segregated in red and green channels or merged for quantification. Quantification can be achieved using Fiji and an image-based script19 that measures fluorescent dextran uptake (or uptake of other fluorescent markers) on a per-cell basis (described in the protocol above) for analysis of individual cells and large numbers of cells in whole populations. This method can gather distinct, relative measurements for dextrans of different colors and sizes within dual-labeled cells, as depicted in Figure 2, to record the different volumes and behaviors of uptake through macropinocytic and endocytic pathways. Quantification of fluorescence intensities is facilitated by having macropinosomes and endosomes sharply delineated against the high contrast black cell backgrounds.
Fluorescent dextrans can also be combined with a myriad of other labeled cargo or membrane proteins or lipids to obtain contextual information for macropinocytosis. By demonstrating examples of fluorescent lipid probes, we show how different stages of macropinocytosis can be defined. Furthermore, it is also possible to combine multiple lipid probes as well as dextran to track the dynamic transitions of phosphoinositides throughout macropinocytosis24. The roles of successive phospholipids in the process of macropinocytosis and for receptor signaling and sorting within macropinosomes have been well documented and can also be manipulated with lipid kinase and phosphatase inhibitors 9,25,26. Another important approach is to compare the uptake of fluid-phase dextran with labeled membrane proteins, either to compare the probes within macropinosomes or to contrast different pathways, as shown in Figure 4. Transferrin and dextran can be readily and simultaneously applied to cells to compare internalization in different pathways. Labeled, membrane-associated Rab GTPases can be expressed in cells and used to label different stages of macropinocytosis, including Rab8a, Rab13 and Rab519,24,27,28, while Rab7 and Lamp1 are useful for tracking macropinosome maturation through to fusion with late endo/lysosomes29,30.
The ability to convert dextrans and other fluorescent markers for labeling at an ultrastructural level is now becoming more common due to the refinement of CLEM techniques, including the availability of gridded coverslips for positioning and identifying cells. Large fluorescent dextran-filled macropinosomes are ideal for CLEM31, and cells can be fixed for EM processing at any time after pulse-labeling with dextran or after live imaging of cells bathed in dextran. Correlative techniques combining fluorescence and scanning EM are also available and are particularly powerful for viewing cell surface ruffles32. Viewing the cell ultrastructure allows further characterization of endosomes through their morphological features and events such as homo-or heterotypical fusion, although membrane tubulation is normally disrupted by fixation.
In conclusion, despite its ancient roots and prevalence as a cellular pathway, macropinocytosis is still incompletely understood. Dextran and other labeled, fluid-phase cargos are vital tools for defining and characterizing macropinocytosis in a range of cells, tissues, and organisms. Improvements and modifications in ways to apply dextran and to image and quantify its uptake are important for comprehensively examining this pathway and defining its intersection with other cellular pathways and organelles. The approaches demonstrated here offer ways to optimize dextran imaging in live cells.
Disclosures
The authors declare no competing financial interests.
Acknowledgements
The authors thank Tatiana Khromykh for her expert technical assistance. Fluorescence imaging was performed in IMB Microscopy incorporating the Cancer Ultrastructure and Function Facility funded by the Australian Cancer Research Foundation; electron microscopy was performed in UQ's Centre for Microscopy and Microanalysis. Funding was received from the National Health and Medical Research Council of Australia (JLS APP1176209) and the Australian Research Council (DP180101910). NDC is supported as a CZI Imaging Scientist by grant number 2020-225648 from the Chan Zuckerberg Initiative DAF, an advised fund of Silicon Valley Community Foundation. YH was supported by a Ph.D. scholarship from the Australian government and funding from the Yulgilbar Alzheimer Research Program. Z-JX was supported by a Chinese Academy of Science scholarship.
Materials
| Name | Company | Catalog Number | Comments |
| 2% Osmium Tetroxide | ProSciTech | EMS19192 | |
| 647-Transferrin | Molecular Probes, Invitrogen | T23366 | |
| BV2 cells | Gift kindly given to us by Dr Liviu Bodea (Queensland Brain Institute) | - | |
| CpG (Class B) B | Integrated DNA Technologies | Custom Order | |
| Dextran Alexa Fluor 488 at 10 kDa MW | Life Technology Australia Pty Ltd | D22910 | |
| Dextran Alexa Fluor 647 at 10 kDa MW | Life Technology Australia Pty Ltd | D22914 | |
| Dextran Oregon Green 488 at 70 kDa MW | Life Technology Australia Pty Ltd | D1818 | |
| Dextran Tetramethylrhodamine at 70 kDa MW | Life Technology Australia Pty Ltd | D7173 | |
| DMEM medium with sodium pyruvate and L-glutamine | Gibco Invitrogen | #11995 | |
| Fetal Calf serum | Interpath Services Pty Ltd | SFBS-F | |
| Glutaraldehyde aqueous solution, EM Grade 25% | ProSciTech | C002 | |
| Jeol 1011 electron microscope | JEOL | - | |
| L-Glutamine | Gibco Invitrogen | 25030081 | |
| Lipofectamine 2000 | Gibco Invitrogen | 11668019 | |
| MatTek Glass Bottom Dish 35 mm, uncoated grid | MatTek Corporation | P35G-2-14-CGRD | |
| MatTek glass bottom dishes 35 mm uncoated | MatTek Corporation | P35G-1.5-14C | |
| MDA-MB 231 cells | ATCC | HTB-26 | |
| Opti-MEM reduced serum medium | Thermo Fisher Scientific | 31985088 | |
| Plasmid mCherry-2XFYVE | Gift kindly given to us by Dr Frederic Meunier (University of Queensland) | - | |
| Plasmid mCherry-PLCδ-PH | Gift kindly given to us by Dr Frederic Meunier (University of Queensland) | - | |
| Raw264.7 cells | ATCC | TIB-71 | |
| RPMI medium 1640,without L-glutamine | Gibco Invitrogen | #21870 | |
| Ultrapure LPS | Jomar Life Research Pte Ltd | TLR-3PELPS | |
| Uranyl Acetate | ProSciTech | C079 | |
| Zeiss inverted LSM880 confocal microscope | Zeiss | - |
References
- King, J. S., Kay, R. R. The origins and evolution of macropinocytosis. Philosophical transactions of the Royal Society of London. Series B, Biological Sciences. 374 (1765), 20180158 (2019).
- Stow, J. L., Hung, Y., Wall, A. A. Macropinocytosis: Insights from immunology and cancer. Current Opinion in Cell Biology. 65, 131-140 (2020).
- Kerr, M. C., Teasdale, R. D. Defining macropinocytosis. Traffic. 10 (4), 364-371 (2009).
- Commisso, C., et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 497 (7451), 633-637 (2013).
- Kim, S. M., et al. PTEN deficiency and AMPK activation promote nutrient scavenging and anabolism in prostate cancer cells. Cancer Discovery. 8 (7), 866-883 (2018).
- Recouvreux, M. V., Commisso, C. Macropinocytosis: A metabolic adaptation to nutrient stress in cancer. Frontiers in Endocrinology (Lausanne). 8, 261 (2017).
- Stow, J. L., Condon, N. D. The cell surface environment for pathogen recognition and entry. Clinical & Translational Immunology. 5 (4), 71 (2016).
- Swanson, J. A., Watts, C. Macropinocytosis. Trends in Cell Biology. 5 (11), 424-428 (1995).
- Buckley, C. M., King, J. S. Drinking problems: mechanisms of macropinosome formation and maturation. FEBS Journal. 284 (22), 3778-3790 (2017).
- Berthiaume, E. P., Medina, C., Swanson, J. A. Molecular size-fractionation during endocytosis in macrophages. Journal of Cell Biology. 129 (4), 989-998 (1995).
- Lin, X. P., Mintern, J. D., Gleeson, P. A. Macropinocytosis in different cell types: Similarities and differences. Membranes (Basel). 10 (8), 177 (2020).
- Pacitto, R., Gaeta, I., Swanson, J. A., Yoshida, S. CXCL12-induced macropinocytosis modulates two distinct pathways to activate mTORC1 in macrophages. Journal of Leukocyte Biology. 101 (3), 683-692 (2017).
- Yoshida, S., Pacitto, R., Yao, Y., Inoki, K., Swanson, J. A. Growth factor signaling to mTORC1 by amino acid-laden macropinosomes. Journal of Cell Biology. 211 (1), 159-172 (2015).
- Chvanov, M., et al. Intracellular rupture, exocytosis and actin interaction of endocytic vacuoles in pancreatic acinar cells: initiating events in acute pancreatitis. Journal of Physiology. 596 (13), 2547-2564 (2018).
- Ballesteros, A., Swartz, K. J. Dextran labeling and uptake in live and functional murine cochlear hair cells. Journal of Visualized Experiments: JoVE. (156), e60769 (2020).
- Weigert, R. Imaging the dynamics of endocytosis in live mammalian tissues. Cold Spring Harbor Perspectives in Biology. 6 (4), 017012 (2014).
- Chen, L., et al. A novel method to image macropinocytosis in vivo. Frontiers in Neuroscience. 12, 324 (2018).
- King, N. P., et al. Soluble NSF attachment protein receptor molecular mimicry by a Legionella pneumophila Dot/Icm effector. Cellular Microbiology. 17 (6), 767-784 (2015).
- Condon, N. D., et al. Macropinosome formation by tent pole ruffling in macrophages. Journal of Cell Biology. 217 (11), 3873-3885 (2018).
- Lemmon, M. A. Pleckstrin homology (PH) domains and phosphoinositides. Biochemical Society Symposium. 74, 81-93 (2007).
- Gillooly, D. J., et al. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO Journal. 19 (17), 4577-4588 (2000).
- Mayle, K. M., Le, A. M., Kamei, D. T. The intracellular trafficking pathway of transferrin. Biochimica et Biophysica Acta. 1820 (3), 264-281 (2012).
- Hacker, U., Albrecht, R., Maniak, M. Fluid-phase uptake by macropinocytosis in Dictyostelium. Journal of Cell Science. 110, 105-112 (1997).
- Wall, A. A., et al. Small GTPase Rab8a-recruited Phosphatidylinositol 3-Kinase gamma regulates signaling and cytokine outputs from endosomal toll-like receptors. Journal of Biological Chemistry. 292 (11), 4411-4422 (2017).
- Bohdanowicz, M., Grinstein, S. Role of phospholipids in endocytosis, phagocytosis, and macropinocytosis. Physiological Reviews. 93 (1), 69-106 (2013).
- Swanson, J. A., Yoshida, S. Macropinosomes as units of signal transduction. Philosophical transactions of the Royal Society of London. Series B, Biological Sciences. 374 (1765), 20180157 (2019).
- Schnatwinkel, C., et al. The Rab5 effector Rabankyrin-5 regulates and coordinates different endocytic mechanisms. PLoS Biology. 2 (9), 261 (2004).
- Yeo, J. C., Wall, A. A., Luo, L., Stow, J. L. Sequential recruitment of Rab GTPases during early stages of phagocytosis. Cellular Logistics. 6 (1), 1140615 (2016).
- Racoosin, E. L., Swanson, J. A. Macropinosome maturation and fusion with tubular lysosomes in macrophages. Journal of Cell Biology. 121 (5), 1011-1020 (1993).
- Dolat, L., Spiliotis, E. T. Septins promote macropinosome maturation and traffic to the lysosome by facilitating membrane fusion. Journal of Cell Biology. 214 (5), 517-527 (2016).
- Kuhn, S., Lopez-Montero, N., Chang, Y. Y., Sartori-Rupp, A., Enninga, J. Imaging macropinosomes during Shigella infections. Methods. , 12-22 (2017).
- Kommnick, C., Lepper, A., Hensel, M. Correlative light and scanning electron microscopy (CLSEM) for analysis of bacterial infection of polarized epithelial cells. Scientific Reports. 9 (1), 17079 (2019).
- Schindelin, J., et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 9 (7), 676-682 (2012).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved