Method Article
Non-Radioactive In Vitro Cardiac Myosin Light Chain Kinase Assays
* These authors contributed equally
In This Article
Summary
This study describes protocols for nonradiometric methods, a bioluminescent ADP detection assay and a phosphate-affinity SDS-PAGE, to determine the kinase activity of cardiac myosin light chain kinase (cMLCK) and the phosphorylation level of its substrate, myosin regulatory light chain (MLC2v).
Abstract
Cardiac-specific myosin regulatory light chain kinase (cMLCK) regulates cardiac sarcomere structure and contractility by phosphorylating the ventricular isoform of the myosin regulatory light chain (MLC2v). MLC2v phosphorylation levels are significantly reduced in failing hearts, indicating the clinical importance of assessing the activity of cMLCK and the phosphorylation level of MLC2v to elucidate the pathogenesis of heart failure. This paper describes nonradioactive methods to assess both the activity of cMLCK and MLC2v phosphorylation levels. In vitro kinase reactions are performed using recombinant cMLCK with recombinant calmodulin and MLC2v in the presence of ATP and calcium at 25 °C, which are followed by either a bioluminescent ADP detection assay or a phosphate-affinity SDS-PAGE. In the representative study, the bioluminescent ADP detection assay showed a strict linear increase of the signal at cMLCK concentrations between 1.25 nM to 25 nM. Phosphate-affinity SDS-PAGE also showed a linear increase of phosphorylated MLC2v in the same cMLCK concentration range. Next, the time-dependency of the reactions was examined at the concentration of 5 nM cMLCK. A bioluminescent ADP detection assay showed a linear increase in the signal during 90 min of the reaction. Similarly, phosphate-affinity SDS-PAGE showed a time-dependent increase of phosphorylated MLC2v. The biochemical parameters of cMLCK for MLC2v were determined by a Michaelis-Menten plot using the bioluminescent ADP detection assay. The Vmax was 1.65 ± 0.10 mol/min/mol kinase and the average Km was around 0.5 USA µM at 25 °C. Next, the activity of wild type and the dilated cardiomyopathy-associated p.Pro639Valfs*15 mutant cMLCK were measured. The bioluminescent ADP detection assay and phosphate-affinity SDS-PAGE correctly detected defects in cMLCK activity and MLC2v phosphorylation, respectively. In conclusion, a combination of the bioluminescent ADP detection assay and the phosphate-affinity SDS-PAGE is a simple, accurate, safe, low-cost, and flexible method to measure cMLCK activity and the phosphorylation level of MLC2v.
Introduction
The cardiac-specific myosin regulatory light chain kinase (cMLCK) encoded by the MYLK3 gene is the kinase predominantly responsible for maintaining the phosphorylation of cardiac ventricular myosin regulatory light chain 2 (MLC2v)1,2. By phosphorylating MLC2v at Ser-15, cMLCK promotes sarcomere organization1 and potentiates cardiac contractility2,3 as a result of increasing cross-bridge formation and therefore an increase in the lever-arm stiffness of myosin II4. Defects in cMLCK activity or reduced levels of MLC2v phosphorylation contribute to the development of heart failure in animal models3,5,6. Thus, cMLCK activity plays critical roles in cardiac contractility in both physiological and pathological conditions by regulating the phosphorylation level of MLC2v.
Dilated cardiomyopathy (DCM) is characterized by systolic dysfunction and an enlarged left ventricular chamber size and is a major cause of congestive heart failure and heart transplantations. So far more than 40 genes have been identified as DCM-causing mutations7. Recently, a novel DCM-associated MYLK3 mutation (p.Pro639Valfs*15) was identified that completely abolishes kinase activity due to truncation of the cMLCK protein at the middle portion of its catalytic domain8. Two cases of familial DCM-associated mutations in MYLK3 showing depressed or abolished cMLCK activity have also been reported9. Thus, depressed or abolished cMLCK activity in familial DCM may contribute to the development of the disease by decreasing MLC2v phosphorylation levels. MLC2v phosphorylation levels are also significantly reduced in failing human hearts even without mutations in MYLK310,11. Thus, the reduction of the MLC2v phosphorylation levels seems to be common in human heart failure, indicating that the assessment of cMLCK activity and MLC2v phosphorylation levels is clinically important. It is necessary to explain how the reduced MLC2v phosphorylation levels contribute to depressed cardiac contractility. Accordingly, assays that measure cMLCK activity and MLC2v phosphorylation levels are extremely important for elucidating the pathogenesis of heart failure.
The classical method for measuring cMLCK activity is a radiometric-based assay that quantifies the incorporation of [γ-32P] from radioactively labelled ATP into MLC2v2. However, due to its hazardous nature it requires special safety and environmental considerations, and the cost of waste disposal is high. In addition, the short half-life of32 Prestricts the flexibility of the radiometric assay. To overcome these drawbacks, alternative nonradiometric protein kinase assay techniques have been developed12. The bioluminescent ADP detection assay developed by Promega Corporation measures ADP generated by the protein kinase reaction without using radioisotopes13. It shows comparable results to the radiometric assay for protein kinases with varying levels of activity13. Because the bioluminescent ADP detection assay measures ADP produced by a kinase reaction, phosphate-affinity SDS-PAGE in parallel with bioluminescent ADP detection assay was used to verify whether MLC2v is actually phosphorylated. Phosphate-affinity SDS-PAGE is a phosphate-affinity electrophoresis technique that can detect changes in the mobility of phosphorylated substrate proteins compared to their nonphosphorylated counterparts14.
This article describes protocols for measuring the activity of cMLCK and the phosphorylation level of its substrate, MLC2v, using nonradioactive methods. After performing an in vitro kinase reaction, both the bioluminescent ADP detection assay and phosphate-affinity SDS-PAGE are employed to calculate biochemical values of MLCK and the phosphorylation level of MCL2v, respectively. Overall, a protocol combining the two nonradioactive kinase assays is valuable for the study of kinases.
Protocol
1. Cloning and purification of recombinant wild type and DCM-associated mutant cMLCK
- Cloning of recombinant cardiac myosin light chain kinase into plasmid
- Design a continuous nucleotide sequence to represent the final plasmid.
- Amplify a DNA fragment coding human MYLK3 (NM_1829493.3) using a standard PCR method. The primer sequences are as follows:
Forward primer: 5’- CACCATGTCAGGAACCTCCAAGGAGAGTCTGGGG -3’
Reverse primer: 5’- TTAGGGAGAAGTTGGAAATTTCCTTAACCT -3’
The melting temperature is 60 °C.
NOTE: The single-stranded overhang sequence (CACC) is necessary to ligate to the TOPO cloning vector. - Introduce a DCM-associated mutant construct by primer-derived mutagenesis. The primer sequences are as follows:
Forward primer: 5’- GTACAAGCCTCGAGAGAAGCTGAAGGTGAAC -3’
Reverse primer: 5’- CTTGAGGTCCAGGTGCAGGATGTAGTGCTGGT -3’
The melting temperature is 60 °C. - Run a 0.8% agarose gel at 135 V for 20 min, and cut out the bands corresponding to the PCR products. Then extract and purify the PCR products using a gel extraction kit (see Materials) following the manufacturer’s instructions.
- Perform a TOPO cloning reaction following the manufacturer’s instructions (see Materials) and transform Escherichia coli (pENTR/cMLCK WT, mutant vector).
- Confirm the correct sequence by PCR using the following universal M13 primers. The primer sequences are as follows:
Forward primer: 5’- GTAAAACGACGGCCAGT -3’
Reverse primer: 5’- GTCATAGCTGTTTCCTG -3’ - Incorporate a FLAG-tag into the pENTR/cMLCK vector and perform a LR recombination reaction between MYLK3 and a GateWay destination vector (i.e., pEF-DEST51) following the manufacturer’s instructions (pEF-DEST51/FLAG-cMLCK WT, mutant).
- Cell culture and transfection into HEK293T cells
- Seed a 100 mm dish with 7.5 x 106 HEK293T cells and culture with DMEM supplemented with 10% FBS and 1% penicillin-streptomycin. Incubate at 37 °C in 5% CO2 for 24 h.
- Prepare the Lipofection reagent/DNA mix for transfection into HEK293T cells by mixing 10 µg of plasmid DNA in 500 µL of MEM (see Table of Materials). At the same time, prepare the mixture by adding 20 µL of Lipofectamine 2000 in 500 µL of MEM.
- Incubate for 5 min at room temperature (RT), then mix the Lipofectamine-DNA solution.
- Incubate for 20 min at RT, then add 1 mL of the Lipofectamine-DNA solution to the 100 mm dish.
NOTE: To avoid detaching the cells, add the lipofection mixture gently. - Incubate the transfected cells for up to 48 h at 37 °C with 5% CO2.
- Purification of recombinant cMLCK protein
- Place the transfected cells on ice and wash 3x with 5 mL of PBS.
- Prepare lysis buffer (50 mM Tris-HCl, 0.15 M NaCl, 1% NP40, 0.5 mM EDTA, 0.5 mM EGTA, and protease inhibitor cocktail, pH = 7.5) and keep on ice.
NOTE: Add the protease inhibitor cocktail just before the assay. - Add 1 mL of lysis buffer to the cells, use a scraper to harvest cells into a 1.5 mL tube, and incubate on ice for 5 min.
- Centrifuge at 20,000 x g for 5 min at 4 °C.
- Collect supernatant in a new 1.5 mL tube and incubate with 5 µL of FLAG-M2 agarose for 1 h at 4 °C.
- Centrifuge at 1,000 x g for 1 min at 4 °C, then remove the supernatant and wash in FLAG-M2 agarose 3x using washing buffer (50 mM Tris-HCl, 0.15 M NaCl, 1% NP40, 0.5 mM EDTA, 0.5 mM EGTA, and protease inhibitor cocktail, pH = 7.5).
- Elute the binding proteins with elution buffer (50 mM Tris-HCl, 0.15 M NaCl, 1% NP40, 0.5 mM EDTA, 0.5 mM EGTA, and protease inhibitor cocktail, pH = 7.5) at 4 °C for 30 min.
- Centrifuge at 1,000 x g at 4 °C for 3 min and use the supernatant as the recombinant FLAG-tagged proteins.
2. Cloning and purification of recombinant calmodulin
- Cloning of recombinant calmodulin into the plasmid
- Design a continuous nucleotide sequence to represent the final plasmid.
- Amplify a DNA fragment coding human calmodulin (CALM1) (NM_006888) using a standard PCR method. The primer sequences are as follows:
Forward primer: 5’- CACCATGGCTGATCAGCTGACCGAAGAACAGATT -3’
Reverse primer: 5’- TCATTTTGCAGTCATCATCTGTACGAATTC -3’.
The melting temperature is 60 °C.
NOTE: The single-stranded overhang sequences (CACC) are necessary for ligating to the TOPO cloning vector. - Run a 0.8% agarose gel at 135 V for 20 min, and cut the bands corresponding to the PCR products out of the gel. Then extract and purify the PCR products using a gel extraction kit (see Materials) following the manufacturer’s instructions.
- Perform the TOPO cloning reaction following the manufacturer’s instructions (see Materials) and transform into E. coli (pENTR/Calmodulin).
- Confirm correct sequence by PCR using the following universal M13 primers. The primer sequenced are as follows:
Forward primer: 5’- GTAAAACGACGGCCAGT -3’
Reverse primer: 5’- GTCATAGCTGTTTCCTG -3’ - Perform the LR recombination reaction between the calmodulin and a GateWay destination vector (pEF-DEST17) following the manufacturer's instructions.
- Transform into BL21 (DE3) chemically competent E. coli.
- Cell culture and induction of expression
- Inoculate 5 mL of LB medium containing 100 µg/mL ampicillin with a colony from the transformed E. coli and shake overnight at 37 °C.
- Transfer to 200 mL of LB medium containing 100 µg/mL ampicillin and incubate at 37 °C until the culture optical density (600nm) reaches 0.5.
- Add arabinose to a final concentration of 0.2% and culture for 3 h at 37 °C.
- Centrifuge at 5,000 x g at 4 °C for 10 min and remove the supernatant.
- Suspend in 10 mL of BugBuster Master Mix containing EDTA-free protease inhibitor cocktail and rotate at RT for 20 min.
- Centrifuge at 16,000 x g at 4 °C for 10 min.
- The resulting supernatant containing the N-terminus His-tagged calmodulin protein was loaded onto a column of TALON Affinity Resin equilibrated with immobilized metal ion chromatography binding buffer.
- Elute the bound His-tagged calmodulin protein with elution buffer (50 mM sodium phosphate [pH = 8.0], 0.3 M NaCl, 0.1% CHAPS, and 0.15 M imidazole), then refold and concentrate by centrifugation at 5,000 x g at 4 °C using a centrifugal filter.
- The protein concentration was adjusted to 10 μM and the solution kept at -80 °C until use.
3. In vitro kinase assay
NOTE: All steps are performed on ice to prevent the kinase reaction from proceeding. Additionally, the MLCK and substrate solutions are mixed separately to avoid an overly fast reaction.
- Prepare the following solutions and keep on ice:
10x kinase buffer (200 mM HEPES, 10 mM CaCl2, 50 mM MgCl2, 0.1% Tween 20, pH = 7.5)
100 mM DTT
10 mM ATP
500 nM calmodulin
100 nM FLAG-tagged cMLCK
24 µM His-tagged MLC2v
NOTE: Recombinant proteins are diluted using 1x kinase buffer according to the indicated concentrations. - Prepare 100 µL of the cMLCK master solution on ice in 1.5 mL tubes using the following ingredients:
10 µL of 10x kinase buffer
20 µL of 100 nM cMLCK
20 µL of 5 µM calmodulin
8 µL of 100 mM DTT
42 µL of H2O
NOTE: Each sample is diluted with 10x kinase buffer and distilled water and kept on ice. - Prepare 30 µL of substrate solution in the 8 strip PCR tube on ice at each MLC2v concentration as described in Table 1. The final MLC2v concentrations are 0, 0.25, 0.5, 1, 2, 4, 8, and 12 µM.
NOTE: Recombinant His-tagged MLC2v is diluted with 10x kinase buffer and distilled water. The volume of substrate solution is adjusted to 30 µL by adding water to obtain the appropriate MLC2v concentration. - Add 10 µL of the MLCK master solution to achieve a final reaction solution volume of 40 µL. The final concentration of each component in the kinase reaction is 20 mM HEPES, 1 mM CaCl2, 5 mM MgCl2, 0.01% Tween 20, 2 mM DTT, 150 μM ATP, 5 nM cMLCK, 250 nM calmodulin, and 0, 0.25, 0.5, 1, 2, 4, 8, or 12 μM MLC2v (pH = 7.5).
- Mix well and spin down.
- Incubate the reaction samples at 25 °C for the indicated time.
- After incubation, measure kinase activity by phosphate-affinity SDS-PAGE or bioluminescent ADP detection assay.
4. Phosphate-affinity SDS-PAGE
NOTE: Phosphate-affinity SDS-PAGE was performed according to the manufacturer’s protocol (see Table of Materials).
- Pour the gel for phosphate-affinity SDS-PAGE.
- Mix stacking gel solutions as follows: 12% wt/vol acrylamide, 0.1% wt/vol SDS, 125 mM Tris-HCl (pH = 6.8), 0.1% wt/vol ammonium persulfate, and 0.5% vol/vol N, N, N’, N’- tetramethylethylenediamine.
- Mix resolving gel solutions as follows: 12% wt/vol acrylamide, 30 µM Phos-tag acrylamide, 60 µM MnCl2, 0.1% wt/vol SDS, 375 mM Tris-HCl (pH = 8.8), 0.05% wt/vol ammonium persulfate, and 0.25% vol/vol N, N, N’, N’- tetramethylethylenediamine.
- Prepare sample for electrophoresis.
- Add and mix 1 mM MnCl2 to the sample from the in vitro kinase reaction.
- Perform electrophoresis and Western blotting.
- Run the gel at 150 V for 80 min in running buffer (0.1% wt/vol SDS, 25 mM Tris, and 192 mM glycine).
- After electrophoresis, soak the gel in EDTA (+) transfer buffer (10 mM EDTA, 50 mM Tris, 380 mM glycine, 0.000375% wt/vol SDS, and 20% wt/vol ethanol) for 10 min and then in transfer buffer for 10 min.
- Transfer proteins to PVDF membrane at 15 V for 30 min in transfer buffer.
- Rock the membrane continuously with non-fat dry milk for 30 min in RT. After blocking, wash the membrane 3x with TTBS (50 mM Tris, 150 mM NaCl, 0.001% Tween).
- Soak the membrane with the primary antibody (anti-MLC2v, 1:4,000; Abcam ab92721) overnight at 4 °C.
- Wash the membrane 3x with TTBS (50 mM Tris, 150 mM NaCl, and 0.001% Tween).
- Soak the membrane with the secondary antibody (HRP-coupled goat anti-rabbit 1:8,000; Cappel, #55696) for 1 h at RT.
- Wash the membrane 3x with TTBS.
- Detect and analyze proteins on the membrane.
- Add ECL (Enhanced Chemi Luminescence reagent) detection reagent to the membrane for 1 min at RT.
- Detect the proteins on the membrane using a LAS-4000 at the optimal time.
- Quantify the phosphorylated and nonphosphorylated MLC2v, subtracting the background densitometry using ImageQuant TL software.
5. Bioluminescent ADP detection assay
NOTE: The bioluminescent ADP detection assay was performed according to the manufacturer’s protocol.
- Stop the kinase reaction by adding 40 µL of ATP depletion reagent into the kinase assay reaction solution. Mix well and incubate for 30 min at RT.
- Add 80 µL of ADP detection reagent. Mix well and incubate for 30 min at RT.
- Measure the luminescence using a luminometer with a suggested maximum integration time of 0.5 s per well.
- Convert the luminescence intensity to ADP concentration using a calibration curve.
- Calculate the amount of phosphates used for MLC2v phosphorylation.
NOTE: The total ADP produced during the kinase reaction is measured as described above. Background ADP, including cMLCK autophosphorylation, is measured based on a reaction without MLC2v, and the amount of ADP used for MLC2v phosphorylation is assessed by subtracting background ADP from total ADP.
6. Data Analysis
- Fit data to the Michalis-Menten equation using appropriate data analysis software. Data are expressed as mean ± standard deviation.
Results
The classical method for measuring kinase activity is a radiometric-based assay that quantifies the radiolabeled phosphate incorporated into the kinase substrate. For the method presented here, a nonradioactive, in vitro cMLCK kinase assay using purified wild type cMLCK (Figure 1A), MLC2v, and calmodulin was developed (Figure 1B), and kinase activity was determined using a bioluminescent ADP detection assay. For the experiments used to establish the cMLCK assay, purified cMLCK from HEK293T cells was used. In order to determine what cMLCK concentration and reaction time guarantee signal linearity, MLC2v was first incubated with seven different concentrations of cMLCK at 25 °C. After 1 h, the kinase activity was measured by the bioluminescent ADP detection assay and the linear assay region was found between 1.25 and 20 nM cMLCK (Figure 1C), which was confirmed by phosphate-affinity SDS-PAGE (Figure 1D). Next, 25 nM cMLCK was incubated for four different durations (15, 30, 60, 90 min) and kinase activities were measured. Strict linearity of the signal during 90 min of the reaction was observed (Figure 1E,1F). Thus, a cMLCK concentration of 5 nM was used to guarantee strict linearity between the assay signal and kinase activity. Next, the values of Km and Vmax for MLC2v of cMLCK were determined in the presence of calmodulin, ATP, CaCl2, and MgCl2. The average Vmax value was 1.65 ± 0.10 mol/min/mol kinase, and the average MLC2v Km value was around 0.49 ± 0.10 µM at 25 °C (Figure 1G). Thus, a nonradioactive, in vitro cMLCK kinase test using a bioluminescent ADP detection assay and phosphate-affinity SDS-PAGE was developed.
Next, the functional consequences of the p.Pro639Valfs*15 mutation of the MYLK3 gene, which is associated with human DCM pathogenesis, was examined. Wild type and mutant cMLCK was purified from HEK293T cells (Figure 2A), and the best fit was obtained using a model that describes cMLCK-MLC2v reactions based on Michaelis-Menten kinetics. Wild type cMLCK had a Km value of 0.64 ± 0.13 μM and Vmax value of 2.15 ± 0.10 mol/min/mol kinase. Mutant cMLCK had no kinase activity. n = 2 for each point. (Figure 2B,2C)
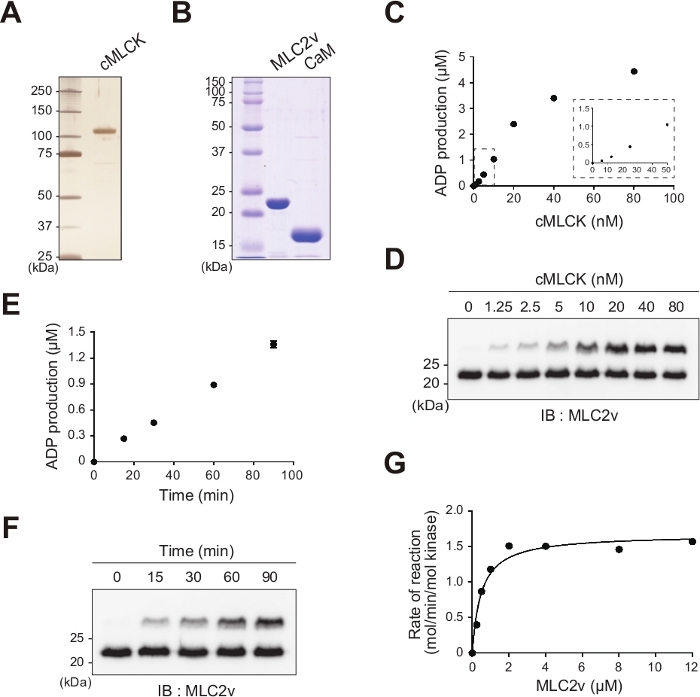
Figure 1: Establishment of nonradioactive, in vitro cMLCK kinase assay system.
(A) Silver staining of recombinant FLAG-tagged wild type human cMLCK proteins from insect cells used in the in vitro kinase assay. (B) Coomassie staining of purified His6-tagged human MLC2v and His-tagged calmodulin from E. coli. (C, D) Different concentrations of cMLCK (0, 1.25, 2.5, 5, 10, 20, 40, and 80 nM) were incubated with 12 µM MLC2v, 150 µM ATP, and 250 nM calmodulin at 25 °C for 1 h, and the kinase activities or MLC2v phosphorylation levels were measured by bioluminescent ADP detection assay (C) or phosphate-affinity SDS-PAGE (D), respectively. Phosphate-affinity SDS-PAGE was followed by immunoblot analysis with an anti-MLC2v antibody. Bands corresponding to phosphorylated and nonphosphorylated MLC2v are marked with open and closed circles, respectively. (E, F) In vitro kinase reactions were performed with 5 nM cMLCK in the presence of 150 µM ATP, 250 nM calmodulin, and 12 µM MLC2v at 25 °C for the indicated times (0, 15, 30, 60, 90 min), and the kinase activity was measured by bioluminescent ADP detection assay (E) or phosphate-affinity SDS-PAGE (F). (G) The MLC2v dose-dependence curve of the cMLCK activity was fitted using the Michaelis-Menten equation. Km (MLC2v) = 0.49 ± 0.10 µM. Vmax = 1.65 ± 0.10 mol/min/mol kinase. Each point represents the mean of duplicate measurements using two different protein preparations. Please click here to view a larger version of this figure.
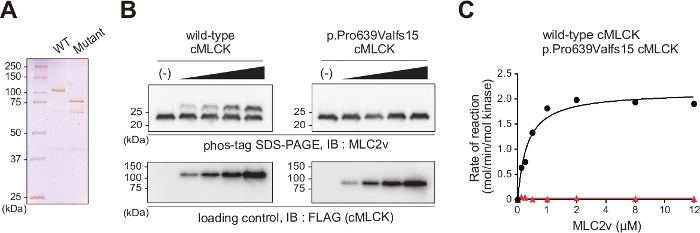
Figure 2: The kinase activities of wild type and DCM-associated mutant cMLCK.
(A) Silver staining of recombinant FLAG-tagged wild type and p.Pro639Valfs*15 mutant human cMLCK proteins from HEK293T cells used in the in vitro kinase assay. (B) Same amounts of four different concentrations of wild type and p.Pro639Valfs*15 mutant cMLCK were incubated with 12 µM MLC2v, 150 µM ATP, and 250 nM calmodulin at 25 °C for 1 h, and the phosphorylation levels of MLC2v were measured by phosphate-affinity SDS-PAGE. Upper panels show phosphate-affinity SDS-PAGE of MLC2v and subsequent immunoblot analysis with anti-MLC2v antibody. Bands corresponding to phosphorylated and nonphosphorylated MLC2v are marked with open and closed circles, respectively. Lower panels show the loading control of purified Flag-tagged cMLCK proteins used in the kinase assays. (C) The MLC2v dose-dependence curves of wild type and p.Pro639Valfs*15 mutant cMLCK activities were fitted using the Michaelis-Menten equation. Wild type cMLCK had a Km value of 0.64 ± 0.13 μM and Vmax value of 2.15 ± 0.10 mol/min/mol kinase. The p.Pro639Valfs*15 mutant had no kinase activity. Each point represents the mean of duplicate measurements using two different protein preparations. RLU, relative light unit. Please click here to view a larger version of this figure.
Discussion
The present study was undertaken to assess whether the combination of nonradioactive methods, the bioluminescent ADP detection assay and the phosphate-affinity SDS-PAGE could successfully be used to determine the activity of cMLCK. It is essential to perform the kinase reactions under the optimal temperature and reaction time. Increasing either of these will rapidly and strongly promote the enzyme reaction. In the present study, the in vitro kinase reaction was performed with 5 nM of cMLCK at 25 °C, which ensured signal linearity for at least 90 min. The bioluminescent ADP detection assay uses a three-step process to quantitate the amount of ADP generated during the protein kinase reaction13. After this reaction is complete, the residual ATP is fully depleted and all ADP produced by the reaction is converted to ATP. Only the newly generated ATP is then used for the kinase activity that generates the luminescence signal. For accurate determination of the biochemical values (e.g., Km of substrate and Vmax), ADP-ATP standard solutions with various ADP/ATP ratios (final concentration of 150 µM) should be prepared in the same volumes as the kinase reaction solutions. In addition, it is important to measure the signal of cMLCK in the absence of MLC2v simultaneously to exclude the ADP from ATP hydrolysis that does not result in phosphorylation of MLC2v. In the present study, bioluminescent ADP detection assay determined the Km for MLC2v value was around 0.5 µM and a Vmax value was around 1.7 to 2.2 mol/min/mol kinase in wild type cMLCK. These values showed minor differences from the values determined by the in vitro radioactive kinase assay2 because of the difference in the reaction temperature. However, the results appear to be within the acceptable range.
The bioluminescent ADP detection assay does not directly observe the phosphorylation level of the protein kinase substrate, while the radiometric assay can observe the direct incorporation of γ-32P into the protein kinase substrate. Accordingly, the phosphate-affinity SDS-PAGE was performed in parallel using the same sample to complement the bioluminescent ADP detection assay that can visualize the phosphorylated substrate as slower migration bands with corresponding nonphosphorylated substrate14. Phosphate-affinity SDS-PAGE showed time- and dose-dependent increases in the amounts of phosphorylated MLC2v, which is consistent with the results of ADP-Glo assay. Furthermore, the validity of both the bioluminescent ADP detection assay and the phosphate-affinity SDS-PAGE were confirmed by the experiments using wild type and p.Pro639Valfs*15 mutant cMLCK purified from HEK293T cells. Because p.Pro639Valfs*15 mutant cMLCK is truncated at the middle portion of its catalytic domain, its kinase activity is likely abolished8. Indeed, phosphate-affinity SDS-PAGE showed complete disappearance of the phosphorylated bands of MLC2v, and the ADP-Glo assay showed complete disruption of the kinase activity of the mutant cMLCK.
The bioluminescent ADP detection assay can determine the biochemical values of kinase activity accurately and will be the next gold standard assay for protein kinase activity13. However, it cannot directly observe substrate phosphorylation and cannot distinguish the ADP product formed during the kinase reaction from those during an ATPase reaction. On the other hand, phosphate-affinity SDS-PAGE can directly observe the phosphorylation level of the protein kinase substrate14, although it cannot determine the biochemical values of kinase activity accurately. Taken together, combining the bioluminescent ADP detection assay and the phosphate-affinity SDS-PAGE will provide the necessary and sufficient information to determine kinase activity by covering for each other’s weaknesses.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported in part by JSPS KAKENHI Grant Number JP17K09578 and JP18H04050.
Materials
| Name | Company | Catalog Number | Comments |
| 30% acrylamide/Bis solution | Bio-Rad | 1610156 | Store at 4℃ |
| acrylamide | Bio-Rad | 1610156 | Store at 4℃ |
| Amicon Ultra-15 | Merck | UFC901008 | |
| ammonium persulfate | Wako | 019-03435 | |
| ampicillin sodium | Wako | 014-23302 | Store at -20℃ |
| BugBuster | Milipore | 71456-4 | Store at 4℃ |
| CaCl2 | Wako | 031-00435 | |
| CHAPS | Dojindo | 349-04722 | Store at 4℃ |
| chemiluminescence imaging analyzer TriStar2 | CBERTHOLD TECHNOLOGIES | LB942-A | |
| dithiothreitol | Wako | 047-08973 | Store at -20℃ |
| ECL (Enhanced Chemi Luminescence) reagent | GE Healthcare | RPN2106 | Mix reagent 1 and reagent 2 in equal amounts |
| EDTA | DOJINDO | 345-01865 | |
| Ethanol | Wako | 057-00456 | |
| FBS | Sigma-Aldrich | 172012-500ML | Store at -20℃ |
| FLAG agarose | Merck | A2220 | Store at -20℃ |
| FLAG peptide | Merck | F3290-4MG | Store at 4℃ |
| GateWay pEF-DEST51 Vector | Invitrogen | 12285011 | Store at -20℃ |
| glycine | Sigma-Aldrich | 12-1210-5 | |
| HEPES | Dojindo | 342-01375 | |
| Igepal CA-630 (NP40) | Sigma-Aldrich | 13021-500ML | |
| Imiadasole | Wako | 095-00015 | |
| L-(+)-Arabinose | Sigma-Aldrich | A3256-25G | Store at -20℃ |
| LAS-4000 | GE Healthcare | 28955810 | |
| LB | Merck | WM841485 824 | |
| Lipofectamine 2000 | Invitrogen | 11668-019 | |
| Manganase (II) Chloride Tetrahydrate | Wako | 134-15302 | |
| MgCl2 | nacalai-tesque | 20909-42 | |
| N, N, N’, N’- tetramethylethylenediamin | Wako | 110-18-9 | |
| NaCl | Wako | 191-01665 | |
| OneShot BL21 AI | Invitrogen | 44-0184 | Store at -80℃ |
| OptiMEM | gibco | 31985-070 | Store at 4℃ |
| PBS | NISSUI PHARMACEUTICAL | 5913 | Store at 4℃ |
| penicillin streptmycin | gibco | 15140-122 | Store at -20℃ |
| pENTR/D-TOPO Cloning Kit | Invitrogen | K240020 | Store at -20℃ |
| Phos-tag Acrylamide | Wako | AAL-107 | Store at 4℃ |
| Promega ADP-Glo | Promega | V9104 | Store at -20℃ |
| protease inhibitor cock-tail | nacalai-tesque | 25955-11 | |
| PVDF membrane | Merck | IPVH00010 | Pore size : 0.45 μm |
| QIAEX II Gel Extraction Kit (150) | QIAGEN | 20021 | |
| SDS | Wako | 191-07145 | |
| sodium phosphate | Wako | 192-02815 | |
| TALON affinity resin | TaKaRa | 635504 | Store at 4℃ |
| Tris | Sigma-Aldrich | T1503-1KG | |
| Tween 20 | Wako | 167-11515 | Store at 4℃ |
| Ultra Pure Agarose | Invitrogen | 16500-500 | |
| Ultra Pure ATP, 100mM | Promega | V703B-C | Store at -20℃ |
| Urea | Sigma-Aldrich | U0631-1KG |
References
- Seguchi, O., et al. A cardiac myosin light chain kinase regulates sarcomere assembly in the vertebrate heart. The Journal of Clinical Investigation. 117 (10), 2812-2824 (2007).
- Chan, J. Y., et al. Identification of cardiac-specific myosin light chain kinase. Circulation Research. 102 (5), 571-580 (2008).
- Scruggs, S. B., et al. Ablation of ventricular myosin regulatory light chain phosphorylation in mice causes cardiac dysfunction in situ and affects neighboring myofilament protein phosphorylation. Journal of Biological Chemistry. 284 (8), 5097-5106 (2009).
- Sheikh, F., et al. Mouse and computational models link Mlc2v dephosphorylation to altered myosin kinetics in early cardiac disease. Journal of Clinical Investigation. 122 (4), 1209-1221 (2012).
- Ding, P., et al. Cardiac myosin light chain kinase is necessary for myosin regulatory light chain phosphorylation and cardiac performance in vivo. Journal of Biological Chemistry. 285 (52), 40819-40829 (2010).
- Warren, S. A., et al. Myosin light chain phosphorylation is critical for adaptation to cardiac stress. Circulation. 126 (22), 2575-2588 (2012).
- Pinto, Y. M., et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC working group on myocardial and pericardial diseases. European Heart Journal. 37 (23), 1850-1858 (2016).
- Hodatsu, A., et al. Impact of cardiac myosin light chain kinase gene mutation on development of dilated cardiomyopathy. ESC Heart Failure. 6 (2), 406-415 (2019).
- Tobita, T., et al. Identification of MYLK3 mutations in familial dilated cardiomyopathy. Scientific Reports. 7 (1), 17495 (2017).
- Van Der Velden, J., et al. Myosin light chain composition in non-failing donor and end-stage failing human ventricular myocardium. Advances in Experimental Medicine and Biology. 538, 3-15 (2004).
- Morano, I. Tuning the human heart molecular motors by myosin light chairs. Journal of Molecular Medicine. 77 (7), 544-555 (1999).
- Fan, F., Wood, K. V. Bioluminescent assays for high-throughput screening. Assay and Drug Development Technologies. 5 (1), 127-136 (2007).
- Sanghera, J., Li, R., Yan, J. Comparison of the Luminescent ADP-Glo Assay to a Standard Radiometric Assay for Measurement of Protein Kinase Activity. ASSAY and Drug Development Technologies. 7 (6), 615-622 (2009).
- Kinoshita, E., Kinoshita-Kikuta, E., Koike, T. Separation and detection of large phosphoproteins using phos-tag sds-page. Nature Protocols. 4 (10), 1513-1521 (2009).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved